A Natural Dietary Supplement with a Combination of Nutrients Prevents Neurodegeneration Induced by a High Fat Diet in Mice
- PMID: 30134549
- PMCID: PMC6165339
- DOI: 10.3390/nu10091130
A Natural Dietary Supplement with a Combination of Nutrients Prevents Neurodegeneration Induced by a High Fat Diet in Mice
Abstract
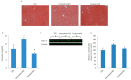
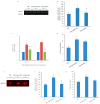
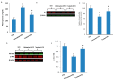
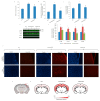
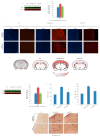
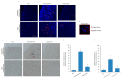
Obesity and metabolic disorders can be risk factors for the onset and development of neurodegenerative diseases. The aim of the present study was to investigate the protective effects of a natural dietary supplement (NDS), containing Curcuma longa, silymarin, guggul, chlorogenic acid and inulin, on dysmetabolism and neurodegeneration in the brains of high fat diet (HFD)-fed mice. Decrease in the expression of FACL-4, CerS-1, CerS-4, cholesterol concentration and increase in the insulin receptor expression and insulin signaling activation, were found in brains of NDS-treated HFD brains in comparison with HFD untreated-mice, suggesting that NDS is able to prevent brain lipid accumulation and central insulin resistance. In the brains of NDS-treated HFD mice, the levels of RNS, ROS and lipid peroxidation, the expression of p-ERK, H-Oxy, i-NOS, HSP60, NF-kB, GFAP, IL-1β, IL-6 and CD4 positive cell infiltration were lower than in untreated HFD mice, suggesting antioxidant and anti-inflammatory effects of NDS. The decreased expression of p-ERK and GFAP in NDS-treated HFD mice was confirmed by immunofluorescence. Lastly, a lower number of apoptotic nuclei was found in cortical sections of NDS-treated HFD mice. The present data indicate that NDS exerts neuroprotective effects in HFD mice by reducing brain fat accumulation, oxidative stress and inflammation and improving brain insulin resistance.
Keywords: HFD mice; insulin resistance; natural antioxidants; neurodegeneration; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

