Vitamin A Deficiency and the Lung
- PMID: 30134568
- PMCID: PMC6164133
- DOI: 10.3390/nu10091132
Vitamin A Deficiency and the Lung
Abstract
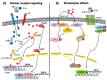
Vitamin A (all-trans-retinol) is a fat-soluble micronutrient which together with its natural derivatives and synthetic analogues constitutes the group of retinoids. They are involved in a wide range of physiological processes such as embryonic development, vision, immunity and cellular differentiation and proliferation. Retinoic acid (RA) is the main active form of vitamin A and multiple genes respond to RA signalling through transcriptional and non-transcriptional mechanisms. Vitamin A deficiency (VAD) is a remarkable public health problem. An adequate vitamin A intake is required in early lung development, alveolar formation, tissue maintenance and regeneration. In fact, chronic VAD has been associated with histopathological changes in the pulmonary epithelial lining that disrupt the normal lung physiology predisposing to severe tissue dysfunction and respiratory diseases. In addition, there are important alterations of the structure and composition of extracellular matrix with thickening of the alveolar basement membrane and ectopic deposition of collagen I. In this review, we show our recent findings on the modification of cell-junction proteins in VAD lungs, summarize up-to-date information related to the effects of chronic VAD in the impairment of lung physiology and pulmonary disease which represent a major global health problem and provide an overview of possible pathways involved.
Keywords: E-cadherin; N-cadherin; Vitamin A deficiency; collagen; epithelial–mesenchymal transition; extracellular matrix; lung; pulmonary disease; retinoic acid; retinol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

