Treatment of acute migraine by a partial rebreathing device: A randomized controlled pilot study
- PMID: 30134739
- PMCID: PMC6158684
- DOI: 10.1177/0333102418797285
Treatment of acute migraine by a partial rebreathing device: A randomized controlled pilot study
Abstract
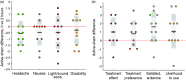
Background Impaired brain oxygen delivery can trigger and exacerbate migraine attacks. Normoxic hypercapnia increases brain oxygen delivery markedly by vasodilation of the cerebral vasculature, and hypercapnia has been shown to abort migraine attacks. Stable normoxic hypercapnia can be induced by a compact partial rebreathing device. This pilot study aimed to provide initial data on the device's efficacy and safety. Methods Using a double-blinded, randomized, cross-over study design, adult migraine-with-aura patients self-administered the partial rebreathing device or a sham device for 20 minutes at the onset of aura symptoms. Results Eleven participants (mean age 35.5, three men) self-treated 41 migraine attacks (20 with the partial rebreathing device, 21 with sham). The partial rebreathing device increased mean End Tidal CO2 by 24%, while retaining mean oxygen saturation above 97%. The primary end point (headache intensity difference between first aura symptoms and two hours after treatment (0-3 scale) - active/sham difference) did not reach statistical significance (-0.55 (95% CI: -1.13-0.04), p = 0.096), whereas the difference in percentage of attacks with pain relief at two hours was significant ( p = 0.043), as was user satisfaction ( p = 0.022). A marked efficacy increase was seen from first to second time use of the partial rebreathing device. No adverse events occurred, and side effects were absent or mild. Conclusion Normoxic hypercapnia shows promise as an adjunctive/alternative migraine treatment, meriting further investigation in a larger population. Clinical study registered at ClinicalTrials.gov with identifier NCT03472417.
Keywords: CO2 therapy; Migraine; headache; hypercapnia; rebreathing.
Figures




References
-
- Olesen J, Friberg L, Skyhoj Olsen T, et al. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann Neurol 1990; 28: 791–798. - PubMed
-
- De Benedittis G. CBF changes during headache-free periods and spontaneous/induced attacks in migraine with and without aura: A TCD and SPECT comparison study. J Neurosurg Sci 1999; 43: 141–147. - PubMed
-
- Sanchez Del Rio M, Bakker D, Wu O, et al. Perfusion weighted imaging during migraine: Spontaneous visual aura and headache. Cephalalgia 1999; 19: 701–707. - PubMed
-
- Denuelle M, Fabre N, Payoux P, et al. Posterior cerebral hypoperfusion in migraine without aura. Cephalalgia 2008; 28: 856–862. - PubMed
-
- Hansen JM, Schytz HW, Larsen VA, et al. Hemiplegic migraine aura begins with cerebral hypoperfusion: Imaging in the acute phase. Headache 2011; 51: 1289–1296. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

