B-1a cells protect mice from sepsis-induced acute lung injury
- PMID: 30134811
- PMCID: PMC6016888
- DOI: 10.1186/s10020-018-0029-2
B-1a cells protect mice from sepsis-induced acute lung injury
Abstract
Background: Sepsis morbidity and mortality are aggravated by acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Mouse B-1a cells are a phenotypically and functionally unique sub-population of B cells, providing immediate protection against infection by releasing natural antibodies and immunomodulatory molecules. We hypothesize that B-1a cells ameliorate sepsis-induced ALI.
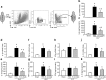
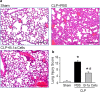
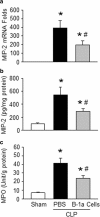
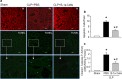
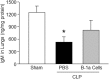
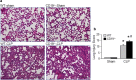
Methods: Sepsis was induced in C57BL/6 mice by cecal ligation and puncture (CLP). PBS or B-1a cells were adoptively transferred into the septic mice intraperitoneally. After 20 h of CLP, lungs were harvested and assessed by PCR and ELISA for pro-inflammatory cytokines (IL-6, IL-1β) and chemokine (MIP-2) expression, by histology for injury, by TUNEL and cleaved caspase-3 for apoptosis, and by myeloperoxidase (MPO) assay for neutrophil infiltration.
Results: We found that septic mice adoptively transferred with B-1a cells significantly decreased the mRNA and protein levels of IL-6, IL-1β and MIP-2 in the lungs compared to PBS-treated mice. Mice treated with B-1a cells showed dramatic improvement in lung injury compared to PBS-treated mice after sepsis. We found apoptosis in the lungs was significantly inhibited in B-1a cell injected mice compared to PBS-treated mice after sepsis. B-1a cell treatment significantly down-regulated MPO levels in the lungs compared to PBS-treated mice in sepsis. The protective outcomes of B-1a cells in ALI was further confirmed by using B-1a cell deficient CD19-/- mice, which showed significant increase in the lung injury scores following sepsis as compared to WT mice.
Conclusions: Our results demonstrate a novel therapeutic potential of B-1a cells to treat sepsis-induced ALI.
Keywords: Acute lung injury; B-1a cells; IL-10; Inflammation; Neutrophils; Sepsis.
Conflict of interest statement
Ethics approval
All animal protocols were approved by our Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Consent for publication
All authors have contributed to, read and approved the final version of this manuscript for submission and publication in the journal
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Senkyunolide I protect against lung injury via inhibiting formation of neutrophil extracellular trap in a murine model of cecal ligation and puncture.Int Immunopharmacol. 2021 Oct;99:107922. doi: 10.1016/j.intimp.2021.107922. Epub 2021 Jul 2. Int Immunopharmacol. 2021. PMID: 34224996
-
Luteolin activates Tregs to promote IL-10 expression and alleviating caspase-11-dependent pyroptosis in sepsis-induced lung injury.Int Immunopharmacol. 2021 Oct;99:107914. doi: 10.1016/j.intimp.2021.107914. Epub 2021 Jul 8. Int Immunopharmacol. 2021. PMID: 34246059
-
Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury.Crit Care. 2015 Feb 26;19(1):53. doi: 10.1186/s13054-015-0782-3. Crit Care. 2015. PMID: 25887405 Free PMC article.
-
Macrophages in sepsis-induced acute lung injury: exosomal modulation and therapeutic potential.Front Immunol. 2025 Jan 7;15:1518008. doi: 10.3389/fimmu.2024.1518008. eCollection 2024. Front Immunol. 2025. PMID: 39840035 Free PMC article. Review.
-
Mechanisms of Sepsis-Induced Acute Lung Injury and Advancements of Natural Small Molecules in Its Treatment.Pharmaceuticals (Basel). 2024 Apr 8;17(4):472. doi: 10.3390/ph17040472. Pharmaceuticals (Basel). 2024. PMID: 38675431 Free PMC article. Review.
Cited by
-
Transcriptomic Analysis Reveals Differential Expression of Genes between Lung Capillary and Post Capillary Venules in Abdominal Sepsis.Int J Mol Sci. 2021 Sep 22;22(19):10181. doi: 10.3390/ijms221910181. Int J Mol Sci. 2021. PMID: 34638535 Free PMC article.
-
Ferroptosis: a key driver and therapeutic target in the pathogenesis of acute respiratory distress syndrome.Front Immunol. 2025 Jul 22;16:1567980. doi: 10.3389/fimmu.2025.1567980. eCollection 2025. Front Immunol. 2025. PMID: 40766305 Free PMC article. Review.
-
CCL25 Inhibition Alleviates Sepsis-Induced Acute Lung Injury and Inflammation.Infect Drug Resist. 2022 Jun 25;15:3309-3321. doi: 10.2147/IDR.S352544. eCollection 2022. Infect Drug Resist. 2022. PMID: 35782530 Free PMC article.
-
Inhibition of visfatin alleviates sepsis-induced intestinal damage by inhibiting Hippo signaling pathway.Inflamm Res. 2022 Aug;71(7-8):911-922. doi: 10.1007/s00011-022-01593-z. Epub 2022 Jun 22. Inflamm Res. 2022. PMID: 35731253 Free PMC article.
-
β-glucan-coupled superparamagnetic iron oxide nanoparticles induce trained immunity to protect mice against sepsis.Theranostics. 2022 Jan 1;12(2):675-688. doi: 10.7150/thno.64874. eCollection 2022. Theranostics. 2022. PMID: 34976207 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

