Gonadotropin-releasing hormone antagonist versus progestin for the prevention of premature luteinising hormone surges in poor responders undergoing in vitro fertilisation treatment: study protocol for a randomised controlled trial
- PMID: 30134964
- PMCID: PMC6106816
- DOI: 10.1186/s13063-018-2850-x
Gonadotropin-releasing hormone antagonist versus progestin for the prevention of premature luteinising hormone surges in poor responders undergoing in vitro fertilisation treatment: study protocol for a randomised controlled trial
Abstract
Background: Progress in vitrification techniques has allowed reproductive physicians to consider new strategies for using progestin as an alternative to a GnRH analogue to improve in vitro fertilisation (IVF). However, the role of progestin in blocking luteinising hormone (LH) surges and its potential in clinical practice are unclear, especially for poor responders. We designed a prospective randomised controlled trial (RCT) to compare the efficacy of a gonadotropin-releasing hormone (GnRH) antagonist and progestin in blocking LH surges and premature ovulation in poor responders.
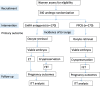
Methods/design: Poor responders who meet the Bologna criteria will be randomised to one of two stimulation regimens-gonadotropin-releasing hormone (GnRH) antagonist or progestin-primed ovarian stimulation (PPOS)-using a computer-generated random number. Fresh embryos were transferred in the GnRH antagonist group and frozen embryos were transferred in the PPOS group. The primary outcome is the incidence of premature LH surges. Secondary outcomes include the number of oocytes retrieved, the number of embryos available for transfer, implantation rates and clinical pregnancy. The sample size for this trial is estimated as 340 participants, with 170 participants in each group. The data analysis will be by intention to treat.
Discussion: To our knowledge, this is the first RCT to examine the efficacy of administering progestin orally to block LH surges and premature ovulation compared with the GnRH antagonist protocols in poor responders undergoing IVF treatment.
Trial registration: www.chictr.org.cn . ChiCTR-IPR-17010906 . Registered on 18 March 2017.
Keywords: GnRH antagonist; LH surge; Poor responders; Progestin.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval has been granted from the Institutional Review Board of Shanghai Ninth People’s Hospital. Written consent will be collected from all participants prior to enrolment.
Consent for publication
Patients will be informed, prior to consenting to participate in the trial, that the results of the study may be presented at academic conferences or published in peer-reviewed journals. Participants will be assured that their confidentiality will be maintained at all times and they will not be identifiable in any publications.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Kuang Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105–111. doi: 10.1016/j.fertnstert.2013.09.007. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

