Drug Hypersensitivity
- PMID: 30135011
- PMCID: PMC6121083
- DOI: 10.3238/arztebl.2018.0501
Drug Hypersensitivity
Abstract
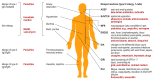
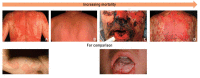
Background: Adverse drug reactions (ADRs) can be divided into pharmacological ADRs (type A) and hypersensitivity reactions (type B). Type B reactions can be further subdivided into immediate (<1 h, urticaria, anaphylaxis) and delayed reactions (>1 h, variable manifestation like exanthema, hepatitis, cytopenias). Prevention of hypersensitivity is often still a challenge.
Methods: Selective literature search in Medline and Google Scholar as well as research in ADR databases like OpenVigil or SIDER.
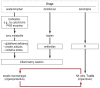
Results: Laboratory tests ([specific] IgE, lymphocyte transformation test), histological examination, dermatological tests (prick tests, epicutaneous testing) and-under certain circumstances-provocation tests can be used for diagnostics. There are only a few pharmacogenetic biomarkers to predict hypersensitivity reactions. Currently, testing for defined HLA genes is mandatory before prescription of abacavir and before the use of carbamazepine in Han Chinese or Thai patients. Immediate discontinuation of the trigger is essential in all allergic hypersensitivity reactions. Immediate reactions are treated with antihistamines, glucocorticoids and occasionally with epinephrine. Delayed reactions are usually treated with glucocorticoids.
Conclusion: Careful, structured diagnostics in case of suspected hypersensitivity together with adequate documentation (allergy passport) is necessary in order to avoid incidents in patients receiving subsequent treatment. Consistent use of existing resources (diagnostics and documentation) can help to avoid hypersensitivity reactions or to rapidly recognize and treat them, respectively.
Figures
Comment in
-
New Classification.Dtsch Arztebl Int. 2018 Oct 19;115(42):713. doi: 10.3238/arztebl.2018.0713a. Dtsch Arztebl Int. 2018. PMID: 30479255 Free PMC article. No abstract available.
References
-
- Doña I, Barrionuevo E, Blanca-Lopez N, et al. Trends in hypersensitivity drug reactions: more drugs, more response patterns, more heterogeneity. J Investig Allergol Clin Immunol. 2014;24:143–153. - PubMed
-
- Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. - PubMed
-
- Jensen CS, Menné T, Lisby S, Kristiansen J, Veien NK. Experimental systemic contact dermatitis from nickel: a dose-response study. Contact Dermatitis. 2003;49:124–132. - PubMed
-
- Dona I, Blanca-Lopez N, Torres MJ, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22:363–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

