Structural and Biochemical Characterization of Endoribonuclease Nsp15 Encoded by Middle East Respiratory Syndrome Coronavirus
- PMID: 30135128
- PMCID: PMC6206473
- DOI: 10.1128/JVI.00893-18
Structural and Biochemical Characterization of Endoribonuclease Nsp15 Encoded by Middle East Respiratory Syndrome Coronavirus
Abstract
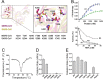
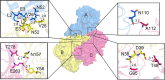
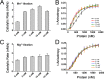
Nonstructural protein 15 (Nsp15) encoded by coronavirus (CoV) is a nidoviral uridylate-specific endoribonuclease (NendoU) that plays an essential role in the life cycle of the virus. Structural information on this crucial protein from the Middle East respiratory syndrome CoV (MERS-CoV), which is lethally pathogenic and has caused severe respiratory diseases worldwide, is lacking. Here, we determined the crystal structure of MERS-CoV Nsp15 at a 2.7-Å resolution and performed the relevant biochemical assays to study how NendoU activity is regulated. Although the overall structure is conserved, MERS-CoV Nsp15 shows unique and novel features compared to its homologs. Serine substitution of residue F285, which harbors an aromatic side chain that disturbs RNA binding compared with that of other homologs, increases catalytic activity. Mutations of residues residing on the oligomerization interfaces that distort hexamerization, namely, N38A, Y58A, and N157A, decrease thermostability, decrease affinity of binding with RNA, and reduce the NendoU activity of Nsp15. In contrast, mutant D39A exhibits increased activity and a higher substrate binding capacity. Importantly, Nsp8 was found to interact with both monomeric and hexameric Nsp15. The Nsp7/Nsp8 complex displays a higher binding affinity for Nsp15. Furthermore, Nsp8 and the Nsp7/Nsp8 complex also enhance the NendoU activity of hexameric Nsp15 in vitro Taking the findings together, this work first provides evidence on how the activity of Nsp15 may be functionally mediated by catalytic residues, oligomeric assembly, RNA binding efficiency, or the possible association with other nonstructural proteins.IMPORTANCE The lethally pathogenic Middle East respiratory syndrome coronavirus (MERS-CoV) and the severe acute respiratory syndrome coronavirus (SARS-CoV) pose serious threats to humans. Endoribonuclease Nsp15 encoded by coronavirus plays an important role in viral infection and pathogenesis. This study determines the structure of MERS-CoV Nsp15 and demonstrates how the catalytic activity of this protein is potentially mediated, thereby providing structural and functional evidence for developing antiviral drugs. We also hypothesize that the primase-like protein Nsp8 and the Nsp7/Nsp8 complex may interact with Nsp15 and affect enzymatic activity. This contributes to the understanding of the association of Nsp15 with the viral replication and transcription machinery.
Keywords: MERS-CoV; crystal structure; endoribonuclease; oligomerization.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- Cavanagh D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol 142:629–633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

