The Novel Activity of Carbamazepine as an Activation Modulator Extends from NaV1.7 Mutations to the NaV1.8-S242T Mutant Channel from a Patient with Painful Diabetic Neuropathy
- PMID: 30135145
- PMCID: PMC7501587
- DOI: 10.1124/mol.118.113076
The Novel Activity of Carbamazepine as an Activation Modulator Extends from NaV1.7 Mutations to the NaV1.8-S242T Mutant Channel from a Patient with Painful Diabetic Neuropathy
Abstract
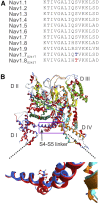
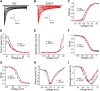
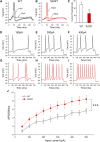
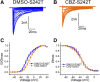
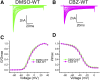
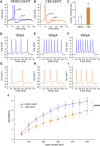
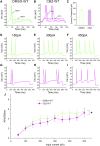
Neuropathic pain in patients carrying sodium channel gain-of-function mutations is generally refractory to pharmacotherapy. However, we have shown that pretreatment of cells with clinically achievable concentration of carbamazepine (CBZ; 30 μM) depolarizes the voltage dependence of activation in some NaV1.7 mutations such as S241T, a novel CBZ mode of action of this drug. CBZ reduces the excitability of dorsal root ganglion (DRG) neurons expressing NaV1.7-S241T mutant channels, and individuals carrying the S241T mutation respond to treatment with CBZ. Whether the novel activation-modulating activity of CBZ is specific to NaV1.7, and whether this pharmacogenomic approach can be extended to other sodium channel subtypes, are not known. We report here the novel NaV1.8-S242T mutation, which corresponds to the NaV1.7-S241T mutation, in a patient with neuropathic pain and diabetic peripheral neuropathy. Voltage-clamp recordings demonstrated hyperpolarized and accelerated activation of NaV1.8-S242T. Current-clamp recordings showed that NaV1.8-S242T channels render DRG neurons hyperexcitable. Structural modeling shows that despite a substantial difference in the primary amino acid sequence of NaV1.7 and NaV1.8, the S242 (NaV1.8) and S241 (NaV1.7) residues have similar position and orientation in the domain I S4-S5 linker of the channel. Pretreatment with a clinically achievable concentration of CBZ corrected the voltage dependence of activation of NaV1.8-S242T channels and reduced DRG neuron excitability as predicted from our pharmacogenomic model. These findings extend the novel activation modulation mode of action of CBZ to a second sodium channel subtype, NaV1.8.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
References
-
- Ahn HS, Dib-Hajj SD, Cox JJ, Tyrrell L, Elmslie FV, Clarke AA, Drenth JP, Woods CG, Waxman SG. (2010) A new Nav1.7 sodium channel mutation I234T in a child with severe pain. Eur J Pain 14:944–950. - PubMed
-
- Akopian AN, Sivilotti L, Wood JN. (1996) A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379:257–262. - PubMed
-
- Ambrósio AF, Soares-Da-Silva P, Carvalho CM, Carvalho AP. (2002) Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem Res 27:121–130. - PubMed
-
- Bennett DL, Woods CG. (2014) Painful and painless channelopathies. Lancet Neurol 13:587–599. - PubMed
-
- Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F, et al. (2012) Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy [published correction appears in Nat Med (2012) 18:1445]. Nat Med 18:926–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

