Cytosolic Processing Governs TAP-Independent Presentation of a Critical Melanoma Antigen
- PMID: 30135181
- PMCID: PMC6457910
- DOI: 10.4049/jimmunol.1701479
Cytosolic Processing Governs TAP-Independent Presentation of a Critical Melanoma Antigen
Abstract
Cancer immunotherapy has been flourishing in recent years with remarkable clinical success. But as more patients are treated, a shadow is emerging that has haunted other cancer therapies: tumors develop resistance. Resistance is often caused by defects in the MHC class I Ag presentation pathway critical for CD8 T cell-mediated tumor clearance. TAP and tapasin, both key players in the pathway, are frequently downregulated in human cancers, correlating with poor patient survival. Reduced dependence on these factors may promote vaccine efficiency by limiting immune evasion. In this study, we demonstrate that PMEL209-217, a promising phase 3 trial-tested antimelanoma vaccine candidate, is robustly presented by various TAP- and/or tapasin-deficient cell lines. This striking characteristic may underlie its potency as a vaccine. Surprisingly, cytosolic proteasomes generate the peptide even for TAP-independent presentation, whereas tripeptidyl peptidase 2 (TPP2) efficiently degrades the epitope. Consequently, inhibiting TPP2 substantially boosts PMEL209-217 presentation, suggesting a possible strategy to improve the therapeutic efficacy of the vaccine.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures
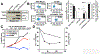





Similar articles
-
Inhibition of serine-peptidase activity enhances the generation of a survivin-derived HLA-A2-presented CTL epitope in colon-carcinoma cells.Scand J Immunol. 2008 Dec;68(6):579-88. doi: 10.1111/j.1365-3083.2008.02175.x. Epub 2008 Oct 22. Scand J Immunol. 2008. PMID: 19000094
-
Targeting tumour cells with defects in the MHC Class I antigen processing pathway with CD8+ T cells specific for hydrophobic TAP- and Tapasin-independent peptides: the requirement for directed access into the ER.Cancer Immunol Immunother. 2007 Aug;56(8):1143-52. doi: 10.1007/s00262-006-0263-2. Epub 2006 Dec 2. Cancer Immunol Immunother. 2007. PMID: 17143611 Free PMC article.
-
ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II.J Immunol. 2009 Feb 1;182(3):1253-9. doi: 10.4049/jimmunol.182.3.1253. J Immunol. 2009. PMID: 19155470
-
Recent Advances in Lung Cancer Immunotherapy: Input of T-Cell Epitopes Associated With Impaired Peptide Processing.Front Immunol. 2019 Jul 3;10:1505. doi: 10.3389/fimmu.2019.01505. eCollection 2019. Front Immunol. 2019. PMID: 31333652 Free PMC article. Review.
-
Proteolytic enzymes involved in MHC class I antigen processing: A guerrilla army that partners with the proteasome.Mol Immunol. 2015 Dec;68(2 Pt A):72-6. doi: 10.1016/j.molimm.2015.04.014. Epub 2015 May 23. Mol Immunol. 2015. PMID: 26006050 Review.
Cited by
-
Uncovering the Tumor Antigen Landscape: What to Know about the Discovery Process.Cancers (Basel). 2020 Jun 23;12(6):1660. doi: 10.3390/cancers12061660. Cancers (Basel). 2020. PMID: 32585818 Free PMC article. Review.
-
Identification of multiple organ metastasis-associated hub mRNA/miRNA signatures in non-small cell lung cancer.Cell Death Dis. 2023 Dec 6;14(12):798. doi: 10.1038/s41419-023-06286-x. Cell Death Dis. 2023. PMID: 38057344 Free PMC article.
-
Aptamers: Cutting edge of cancer therapies.Mol Ther. 2021 Aug 4;29(8):2396-2411. doi: 10.1016/j.ymthe.2021.06.010. Epub 2021 Jun 17. Mol Ther. 2021. PMID: 34146729 Free PMC article. Review.
-
nuTCRacker: Predicting the Recognition of HLA-I-Peptide Complexes by αβTCRs for Unseen Peptides.Eur J Immunol. 2025 Jul;55(7):e51607. doi: 10.1002/eji.202451607. Eur J Immunol. 2025. PMID: 40629982 Free PMC article.
-
Mechanistic diversity in MHC class I antigen recognition.Biochem J. 2021 Dec 22;478(24):4187-4202. doi: 10.1042/BCJ20200910. Biochem J. 2021. PMID: 34940832 Free PMC article. Review.
References
-
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, and Ribas A. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

