Peptide-MHC (pMHC) binding to a human antiviral T cell receptor induces long-range allosteric communication between pMHC- and CD3-binding sites
- PMID: 30135211
- PMCID: PMC6187629
- DOI: 10.1074/jbc.RA118.003832
Peptide-MHC (pMHC) binding to a human antiviral T cell receptor induces long-range allosteric communication between pMHC- and CD3-binding sites
Abstract
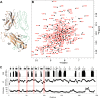
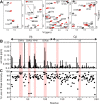
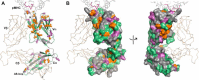
T cells generate adaptive immune responses mediated by the T cell receptor (TCR)-CD3 complex comprising an αβ TCR heterodimer noncovalently associated with three CD3 dimers. In early T cell activation, αβ TCR engagement by peptide-major histocompatibility complex (pMHC) is first communicated to the CD3 signaling apparatus of the TCR-CD3 complex, but the underlying mechanism is incompletely understood. It is possible that pMHC binding induces allosteric changes in TCR conformation or dynamics that are then relayed to CD3. Here, we carried out NMR analysis and molecular dynamics (MD) simulations of both the α and β chains of a human antiviral TCR (A6) that recognizes the Tax antigen from human T cell lymphotropic virus-1 bound to the MHC class I molecule HLA-A2. We observed pMHC-induced NMR signal perturbations in the TCR variable (V) domains that propagated to three distinct sites in the constant (C) domains: 1) the Cβ FG loop projecting from the Vβ/Cβ interface; 2) a cluster of Cβ residues near the Cβ αA helix, a region involved in interactions with CD3; and 3) the Cα AB loop at the membrane-proximal base of the TCR. A biological role for each of these allosteric sites is supported by previous mutational and functional studies of TCR signaling. Moreover, the pattern of long-range, ligand-induced changes in TCR A6 revealed by NMR was broadly similar to that predicted by the MD simulations. We propose that the unique structure of the TCR β chain enables allosteric communication between the TCR-binding sites for pMHC and CD3.
Keywords: T cell activation; T cell receptor (TCR); adaptive immunity; allosteric regulation; conformational change; major histocompatibility complex (MHC); molecular dynamics; nuclear magnetic resonance (NMR); signaling.
© 2018 Rangarajan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
The structural basis of T-cell receptor (TCR) activation: An enduring enigma.J Biol Chem. 2020 Jan 24;295(4):914-925. doi: 10.1074/jbc.REV119.009411. Epub 2019 Dec 17. J Biol Chem. 2020. PMID: 31848223 Free PMC article. Review.
-
Peptide-MHC Binding Reveals Conserved Allosteric Sites in MHC Class I- and Class II-Restricted T Cell Receptors (TCRs).J Mol Biol. 2020 Dec 4;432(24):166697. doi: 10.1016/j.jmb.2020.10.031. Epub 2020 Nov 4. J Mol Biol. 2020. PMID: 33157083 Free PMC article.
-
The Full Model of the pMHC-TCR-CD3 Complex: A Structural and Dynamical Characterization of Bound and Unbound States.Cells. 2022 Feb 14;11(4):668. doi: 10.3390/cells11040668. Cells. 2022. PMID: 35203317 Free PMC article.
-
Identification of the Docking Site for CD3 on the T Cell Receptor β Chain by Solution NMR.J Biol Chem. 2015 Aug 7;290(32):19796-805. doi: 10.1074/jbc.M115.663799. Epub 2015 Jun 24. J Biol Chem. 2015. PMID: 26109064 Free PMC article.
-
Not just another Fab: the crystal structure of a TcR-MHC-peptide complex.Structure. 1997 Feb 15;5(2):159-63. doi: 10.1016/s0969-2126(97)00175-5. Structure. 1997. PMID: 9032079 Review.
Cited by
-
Mechanical force matters in early T cell activation.Proc Natl Acad Sci U S A. 2024 Sep 10;121(37):e2404748121. doi: 10.1073/pnas.2404748121. Epub 2024 Sep 6. Proc Natl Acad Sci U S A. 2024. PMID: 39240966 Free PMC article.
-
Asymmetric framework motion of TCRαβ controls load-dependent peptide discrimination.Elife. 2024 Jan 3;13:e91881. doi: 10.7554/eLife.91881. Elife. 2024. PMID: 38167271 Free PMC article.
-
Mechanical control of antigen detection and discrimination by T and B cell receptors.Biophys J. 2024 Aug 6;123(15):2234-2255. doi: 10.1016/j.bpj.2024.05.020. Epub 2024 May 23. Biophys J. 2024. PMID: 38794795 Free PMC article. Review.
-
The structural basis of T-cell receptor (TCR) activation: An enduring enigma.J Biol Chem. 2020 Jan 24;295(4):914-925. doi: 10.1074/jbc.REV119.009411. Epub 2019 Dec 17. J Biol Chem. 2020. PMID: 31848223 Free PMC article. Review.
-
Multi-scale simulations of the T cell receptor reveal its lipid interactions, dynamics and the arrangement of its cytoplasmic region.PLoS Comput Biol. 2021 Jul 19;17(7):e1009232. doi: 10.1371/journal.pcbi.1009232. eCollection 2021 Jul. PLoS Comput Biol. 2021. PMID: 34280187 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

