Wilms' tumor 1 drives fibroproliferation and myofibroblast transformation in severe fibrotic lung disease
- PMID: 30135315
- PMCID: PMC6141179
- DOI: 10.1172/jci.insight.121252
Wilms' tumor 1 drives fibroproliferation and myofibroblast transformation in severe fibrotic lung disease
Abstract
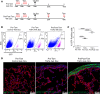
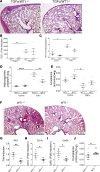
Wilms' tumor 1 (WT1) is a critical transcriptional regulator of mesothelial cells during lung development but is downregulated in postnatal stages and adult lungs. We recently showed that WT1 is upregulated in both mesothelial cells and mesenchymal cells in the pathogenesis of idiopathic pulmonary fibrosis (IPF), a fatal fibrotic lung disease. Although WT1-positive cell accumulation leading to severe fibrotic lung disease has been studied, the role of WT1 in fibroblast activation and pulmonary fibrosis remains elusive. Here, we show that WT1 functions as a positive regulator of fibroblast activation, including fibroproliferation, myofibroblast transformation, and extracellular matrix (ECM) production. Chromatin immunoprecipitation experiments indicate that WT1 binds directly to the promoter DNA sequence of α-smooth muscle actin (αSMA) to induce myofibroblast transformation. In support, the genetic lineage tracing identifies WT1 as a key driver of mesothelial-to-myofibroblast and fibroblast-to-myofibroblast transformation. Importantly, the partial loss of WT1 was sufficient to attenuate myofibroblast accumulation and pulmonary fibrosis in vivo. Further, our coculture studies show that WT1 upregulation leads to non-cell autonomous effects on neighboring cells. Thus, our data uncovered a pathogenic role of WT1 in IPF by promoting fibroblast activation in the peripheral areas of the lung and as a target for therapeutic intervention.
Keywords: Extracellular matrix; Fibrosis; Pulmonology; Respiration.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

