Membrane Active Peptides and Their Biophysical Characterization
- PMID: 30135402
- PMCID: PMC6164437
- DOI: 10.3390/biom8030077
Membrane Active Peptides and Their Biophysical Characterization
Abstract
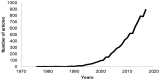
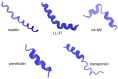
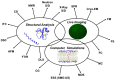
In the last 20 years, an increasing number of studies have been reported on membrane active peptides. These peptides exert their biological activity by interacting with the cell membrane, either to disrupt it and lead to cell lysis or to translocate through it to deliver cargos into the cell and reach their target. Membrane active peptides are attractive alternatives to currently used pharmaceuticals and the number of antimicrobial peptides (AMPs) and peptides designed for drug and gene delivery in the drug pipeline is increasing. Here, we focus on two most prominent classes of membrane active peptides; AMPs and cell-penetrating peptides (CPPs). Antimicrobial peptides are a group of membrane active peptides that disrupt the membrane integrity or inhibit the cellular functions of bacteria, virus, and fungi. Cell penetrating peptides are another group of membrane active peptides that mainly function as cargo-carriers even though they may also show antimicrobial activity. Biophysical techniques shed light on peptide⁻membrane interactions at higher resolution due to the advances in optics, image processing, and computational resources. Structural investigation of membrane active peptides in the presence of the membrane provides important clues on the effect of the membrane environment on peptide conformations. Live imaging techniques allow examination of peptide action at a single cell or single molecule level. In addition to these experimental biophysical techniques, molecular dynamics simulations provide clues on the peptide⁻lipid interactions and dynamics of the cell entry process at atomic detail. In this review, we summarize the recent advances in experimental and computational investigation of membrane active peptides with particular emphasis on two amphipathic membrane active peptides, the AMP melittin and the CPP pVEC.
Keywords: antimicrobial peptides; biophysical characterization; cell-penetrating peptides; membrane disruption; peptide–lipid interactions; uptake mechanism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Cationic membrane peptides: atomic-level insight of structure-activity relationships from solid-state NMR.Amino Acids. 2013 Mar;44(3):821-33. doi: 10.1007/s00726-012-1421-9. Epub 2012 Oct 30. Amino Acids. 2013. PMID: 23108593 Free PMC article. Review.
-
Biophysical properties of membrane-active peptides based on micelle modeling: a case study of cell-penetrating and antimicrobial peptides.J Phys Chem B. 2010 Nov 4;114(43):13726-35. doi: 10.1021/jp1069362. J Phys Chem B. 2010. PMID: 20939546
-
Antimicrobial and cell-penetrating peptides: structure, assembly and mechanisms of membrane lysis via atomistic and coarse-grained molecular dynamics simulations.Protein Pept Lett. 2010 Nov;17(11):1313-27. doi: 10.2174/0929866511009011313. Protein Pept Lett. 2010. PMID: 20673230 Review.
-
Insights into membrane translocation of the cell-penetrating peptide pVEC from molecular dynamics calculations.J Biomol Struct Dyn. 2016 Nov;34(11):2387-98. doi: 10.1080/07391102.2015.1117396. Epub 2016 Mar 17. J Biomol Struct Dyn. 2016. PMID: 26569019
-
How Membrane-Active Peptides Get into Lipid Membranes.Acc Chem Res. 2016 Jun 21;49(6):1130-8. doi: 10.1021/acs.accounts.6b00074. Epub 2016 May 17. Acc Chem Res. 2016. PMID: 27187572 Review.
Cited by
-
The Unusual Aggregation and Fusion Activity of the Antimicrobial Peptide W-BP100 in Anionic Vesicles.Membranes (Basel). 2023 Jan 21;13(2):138. doi: 10.3390/membranes13020138. Membranes (Basel). 2023. PMID: 36837642 Free PMC article.
-
Cancer targeting peptides.Cell Mol Life Sci. 2019 Jun;76(11):2171-2183. doi: 10.1007/s00018-019-03061-0. Epub 2019 Mar 15. Cell Mol Life Sci. 2019. PMID: 30877335 Free PMC article. Review.
-
Structure, Function, and Physicochemical Properties of Pore-forming Antimicrobial Peptides.Curr Pharm Biotechnol. 2024;25(8):1041-1057. doi: 10.2174/0113892010194428231017051836. Curr Pharm Biotechnol. 2024. PMID: 37921126 Review.
-
Characterization of Novel Plantaricin-Derived Antiviral Peptides Against Flaviviruses.Int J Mol Sci. 2025 Jan 25;26(3):1038. doi: 10.3390/ijms26031038. Int J Mol Sci. 2025. PMID: 39940807 Free PMC article.
-
Structural Analysis and Activity Correlation of Amphiphilic Cyclic Antimicrobial Peptides Derived from the [W4R4] Scaffold.Molecules. 2023 Dec 12;28(24):8049. doi: 10.3390/molecules28248049. Molecules. 2023. PMID: 38138539 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

