Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies
- PMID: 30135514
- PMCID: PMC6105651
- DOI: 10.1038/s41467-018-05482-0
Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies
Abstract
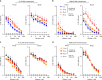
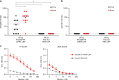
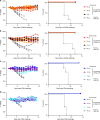
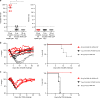
Currently available influenza virus vaccines have inadequate effectiveness and are reformulated annually due to viral antigenic drift. Thus, development of a vaccine that confers long-term protective immunity against antigenically distant influenza virus strains is urgently needed. The highly conserved influenza virus hemagglutinin (HA) stalk represents one of the potential targets of broadly protective/universal influenza virus vaccines. Here, we evaluate a potent broadly protective influenza virus vaccine candidate that uses nucleoside-modified and purified mRNA encoding full-length influenza virus HA formulated in lipid nanoparticles (LNPs). We demonstrate that immunization with HA mRNA-LNPs induces antibody responses against the HA stalk domain of influenza virus in mice, rabbits, and ferrets. The HA stalk-specific antibody response is associated with protection from homologous, heterologous, and heterosubtypic influenza virus infection in mice.
Conflict of interest statement
In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that K.K. and D.W. are named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins. D.W. and N.P. are also named on a patent describing the use of modified mRNA in lipid nanoparticles as a vaccine platform. We have disclosed those interests fully to the University of Pennsylvania, and we have in place an approved plan for managing any potential conflicts arising from licensing of our patents. S.E.H. has received consultancy fee from Lumen, Novavax, and Merck for work unrelated to this report. The remaining authors declare no competing interests.
Figures


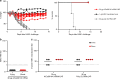
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007324/AI/NIAID NIH HHS/United States
- 1R01AI113047/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- HHSN272201400008C/AI/NIAID NIH HHS/United States
- 1R01AI108686/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- R01-AI124429/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- R01 AI108686/AI/NIAID NIH HHS/United States
- AI109946/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- R01 AI050484/AI/NIAID NIH HHS/United States
- R01 AI113047/AI/NIAID NIH HHS/United States
- HHSN272201400005C/AI/NIAID NIH HHS/United States
- Pathogenesis of Infectious Disease Award/Burroughs Wellcome Fund (BWF)/International
- R01-AI084860/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- New Frontiers/Takeda Pharmaceuticals U.S.A. (Takeda Pharmaceuticals U.S.A., Inc.)/International
- R01 AI124429/AI/NIAID NIH HHS/United States
- U19 AI109946/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

