Experience-dependent structural plasticity targets dynamic filopodia in regulating dendrite maturation and synaptogenesis
- PMID: 30135566
- PMCID: PMC6105721
- DOI: 10.1038/s41467-018-05871-5
Experience-dependent structural plasticity targets dynamic filopodia in regulating dendrite maturation and synaptogenesis
Abstract
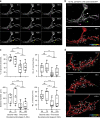
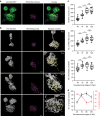
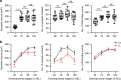
Highly motile dendritic protrusions are hallmarks of developing neurons. These exploratory filopodia sample the environment and initiate contacts with potential synaptic partners. To understand the role for dynamic filopodia in dendrite morphogenesis and experience-dependent structural plasticity, we analyzed dendrite dynamics, synapse formation, and dendrite volume expansion in developing ventral lateral neurons (LNvs) of the Drosophila larval visual circuit. Our findings reveal the temporal coordination between heightened dendrite dynamics with synaptogenesis in LNvs and illustrate the strong influence imposed by sensory experience on the prevalence of dendritic filopodia, which regulate the formation of synapses and the expansion of dendritic arbors. Using genetic analyses, we further identified Amphiphysin (Amph), a BAR (Bin/Amphiphysin/Rvs) domain-containing protein as a required component for tuning the dynamic state of LNv dendrites and promoting dendrite maturation. Taken together, our study establishes dynamic filopodia as the key cellular target for experience-dependent regulation of dendrite development.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Time-lapse Live Imaging and Quantification of Fast Dendritic Branch Dynamics in Developing Drosophila Neurons.J Vis Exp. 2019 Sep 25;(151). doi: 10.3791/60287. J Vis Exp. 2019. PMID: 31609313
-
Proteins that promote filopodia stability, but not number, lead to more axonal-dendritic contacts.PLoS One. 2011 Mar 7;6(3):e16998. doi: 10.1371/journal.pone.0016998. PLoS One. 2011. PMID: 21408225 Free PMC article.
-
Transmembrane agrin regulates dendritic filopodia and synapse formation in mature hippocampal neuron cultures.Neuroscience. 2009 Sep 29;163(1):168-79. doi: 10.1016/j.neuroscience.2009.06.012. Epub 2009 Jun 10. Neuroscience. 2009. PMID: 19524020 Free PMC article.
-
[On the function of dendritic filopodia].Rev Neurol. 2001 Dec 16-31;33(12):1158-66. Rev Neurol. 2001. PMID: 11785056 Review. Spanish.
-
Mechanisms underlying the initiation and dynamics of neuronal filopodia: from neurite formation to synaptogenesis.Int Rev Cell Mol Biol. 2013;301:95-156. doi: 10.1016/B978-0-12-407704-1.00003-8. Int Rev Cell Mol Biol. 2013. PMID: 23317818 Review.
Cited by
-
Maintenance of cell type-specific connectivity and circuit function requires Tao kinase.Nat Commun. 2019 Aug 5;10(1):3506. doi: 10.1038/s41467-019-11408-1. Nat Commun. 2019. PMID: 31383864 Free PMC article.
-
Genome-Wide Association Study of Cerebral Microbleeds on MRI.Neurotox Res. 2020 Jan;37(1):146-155. doi: 10.1007/s12640-019-00073-3. Epub 2019 Jun 18. Neurotox Res. 2020. PMID: 31209788
-
The role of astrocyte-mediated plasticity in neural circuit development and function.Neural Dev. 2021 Jan 7;16(1):1. doi: 10.1186/s13064-020-00151-9. Neural Dev. 2021. PMID: 33413602 Free PMC article. Review.
-
Hippocampal Nogo66-NgR1 signaling activation restricts postsynaptic assembly in aged mice with postoperative neurocognitive disorders.Aging Cell. 2025 Jan;24(1):e14366. doi: 10.1111/acel.14366. Epub 2024 Oct 16. Aging Cell. 2025. PMID: 39412367 Free PMC article.
-
Comparative Connectomics Reveals How Partner Identity, Location, and Activity Specify Synaptic Connectivity in Drosophila.Neuron. 2021 Jan 6;109(1):105-122.e7. doi: 10.1016/j.neuron.2020.10.004. Epub 2020 Oct 28. Neuron. 2021. PMID: 33120017 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

