Structural conservation in a membrane-enveloped filamentous virus infecting a hyperthermophilic acidophile
- PMID: 30135568
- PMCID: PMC6105669
- DOI: 10.1038/s41467-018-05684-6
Structural conservation in a membrane-enveloped filamentous virus infecting a hyperthermophilic acidophile
Abstract
Different forms of viruses that infect archaea inhabiting extreme environments continue to be discovered at a surprising rate, suggesting that the current sampling of these viruses is sparse. We describe here Sulfolobus filamentous virus 1 (SFV1), a membrane-enveloped virus infecting Sulfolobus shibatae. The virus encodes two major coat proteins which display no apparent sequence similarity with each other or with any other proteins in databases. We have used cryo-electron microscopy at 3.7 Å resolution to show that these two proteins form a nearly symmetrical heterodimer, which wraps around A-form DNA, similar to what has been shown for SIRV2 and AFV1, two other archaeal filamentous viruses. The thin (∼ 20 Å) membrane of SFV1 is mainly archaeol, a lipid species that accounts for only 1% of the host lipids. Our results show how relatively conserved structural features can be maintained across evolution by both proteins and lipids that have diverged considerably.
Conflict of interest statement
The authors declare no competing interests.
Figures




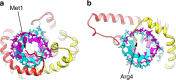

Similar articles
-
Structures of filamentous viruses infecting hyperthermophilic archaea explain DNA stabilization in extreme environments.Proc Natl Acad Sci U S A. 2020 Aug 18;117(33):19643-19652. doi: 10.1073/pnas.2011125117. Epub 2020 Aug 5. Proc Natl Acad Sci U S A. 2020. PMID: 32759221 Free PMC article.
-
A Novel Type of Polyhedral Viruses Infecting Hyperthermophilic Archaea.J Virol. 2017 Jun 9;91(13):e00589-17. doi: 10.1128/JVI.00589-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424284 Free PMC article.
-
A packing for A-form DNA in an icosahedral virus.Proc Natl Acad Sci U S A. 2019 Nov 5;116(45):22591-22597. doi: 10.1073/pnas.1908242116. Epub 2019 Oct 21. Proc Natl Acad Sci U S A. 2019. PMID: 31636205 Free PMC article.
-
Structure and cell biology of archaeal virus STIV.Curr Opin Virol. 2012 Apr;2(2):122-7. doi: 10.1016/j.coviro.2012.01.007. Epub 2012 Mar 20. Curr Opin Virol. 2012. PMID: 22482708 Free PMC article. Review.
-
Genetics, biochemistry and structure of the archaeal virus STIV.Biochem Soc Trans. 2009 Feb;37(Pt 1):114-7. doi: 10.1042/BST0370114. Biochem Soc Trans. 2009. PMID: 19143613 Review.
Cited by
-
Principles for enhancing virus capsid capacity and stability from a thermophilic virus capsid structure.Nat Commun. 2019 Oct 2;10(1):4471. doi: 10.1038/s41467-019-12341-z. Nat Commun. 2019. PMID: 31578335 Free PMC article.
-
Mechanical tomography of an archaeal lemon-shaped virus reveals membrane-like fluidity of the capsid and liquid nucleoprotein cargo.Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2307717120. doi: 10.1073/pnas.2307717120. Epub 2023 Oct 12. Proc Natl Acad Sci U S A. 2023. PMID: 37824526 Free PMC article.
-
New virus isolates from Italian hydrothermal environments underscore the biogeographic pattern in archaeal virus communities.ISME J. 2020 Jul;14(7):1821-1833. doi: 10.1038/s41396-020-0653-z. Epub 2020 Apr 22. ISME J. 2020. PMID: 32322010 Free PMC article.
-
A filamentous archaeal virus is enveloped inside the cell and released through pyramidal portals.Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2105540118. doi: 10.1073/pnas.2105540118. Proc Natl Acad Sci U S A. 2021. PMID: 34341107 Free PMC article.
-
Selective lipid recruitment by an archaeal DPANN symbiont from its host.Nat Commun. 2024 Apr 22;15(1):3405. doi: 10.1038/s41467-024-47750-2. Nat Commun. 2024. PMID: 38649682 Free PMC article.
References
-
- Beijerinck MW. Over een contagium vivum fluidum als oorzaak van de vlekziekte der tabaksbladen. Versl. Gew. Verg. Wis. En. Nat. Afd. 1898;7:229–235.
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA044579/CA/NCI NIH HHS/United States
- R35GM122510/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
- G20 RR031199/RR/NCRR NIH HHS/United States
- R35 GM122510/GM/NIGMS NIH HHS/United States
- S10 OD018149/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

