Parkin and PINK1 mitigate STING-induced inflammation
- PMID: 30135585
- PMCID: PMC7362342
- DOI: 10.1038/s41586-018-0448-9
Parkin and PINK1 mitigate STING-induced inflammation
Retraction in
-
Retraction Note: Parkin and PINK1 mitigate STING-induced inflammation.Nature. 2025 Aug;644(8078):1116. doi: 10.1038/s41586-025-09346-8. Nature. 2025. PMID: 40739362 No abstract available.
Abstract
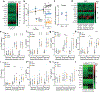
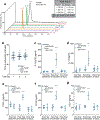
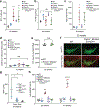
Although serum from patients with Parkinson's disease contains elevated levels of numerous pro-inflammatory cytokines including IL-6, TNF, IL-1β, and IFNγ, whether inflammation contributes to or is a consequence of neuronal loss remains unknown1. Mutations in parkin, an E3 ubiquitin ligase, and PINK1, a ubiquitin kinase, cause early onset Parkinson's disease2,3. Both PINK1 and parkin function within the same biochemical pathway and remove damaged mitochondria from cells in culture and in animal models via mitophagy, a selective form of autophagy4. The in vivo role of mitophagy, however, is unclear, partly because mice that lack either PINK1 or parkin have no substantial Parkinson's-disease-relevant phenotypes5-7. Mitochondrial stress can lead to the release of damage-associated molecular patterns (DAMPs) that can activate innate immunity8-12, suggesting that mitophagy may mitigate inflammation. Here we report a strong inflammatory phenotype in both Prkn-/- and Pink1-/- mice following exhaustive exercise and in Prkn-/-;mutator mice, which accumulate mutations in mitochondrial DNA (mtDNA)13,14. Inflammation resulting from either exhaustive exercise or mtDNA mutation is completely rescued by concurrent loss of STING, a central regulator of the type I interferon response to cytosolic DNA15,16. The loss of dopaminergic neurons from the substantia nigra pars compacta and the motor defect observed in aged Prkn-/-;mutator mice are also rescued by loss of STING, suggesting that inflammation facilitates this phenotype. Humans with mono- and biallelic PRKN mutations also display elevated cytokines. These results support a role for PINK1- and parkin-mediated mitophagy in restraining innate immunity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


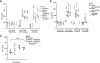




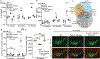
Comment in
-
Pink1/Parkin link inflammation, mitochondrial stress, and neurodegeneration.J Cell Biol. 2018 Oct 1;217(10):3327-3329. doi: 10.1083/jcb.201808118. Epub 2018 Aug 28. J Cell Biol. 2018. PMID: 30154188 Free PMC article.
-
Elusive mitochondrial connection to inflammation uncovered.Nature. 2018 Sep;561(7722):185-186. doi: 10.1038/d41586-018-05988-z. Nature. 2018. PMID: 30194366 No abstract available.
References
-
- Dzamko N, Geczy CL & Halliday GM Inflammation is genetically implicated in Parkinson’s disease. Neurosci. 302, 89–102 (2015) - PubMed
-
- Kitada T et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998) - PubMed
-
- Valente EM et al. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann. Neurol 56, 336–341 (2004) - PubMed
-
- Goldberg MS et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem 278, 43628–43635 (2003) - PubMed
REFERENCES pertaining to Materials and Methods
-
- Kujoth GC, et al. Mitochondrial DNA mutations, oxidative stress and apoptosis in mammalian aging. Science 309:481–484 (2005) - PubMed
-
- Horder M et al. International Federation of Clinical Chemistry, Scientific Division Committee on Enzymes: approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes. Part 7. IFCC method for creatine kinase (ATP: creatine N-phosphotransferase, EC 2.7.3.2). Eur. J. Clin. Chem. Clin. Biochem 29, 435–456 (1991) - PubMed
-
- Ye W et al. Accurate quantitation of circulating cell-free mitochondrial DNA in plasma by droplet digital PCR. Anal. Bioanal. Chem 409, 2727–2735 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

