Stress Odorant Sensory Response Dysfunction in Drosophila Fragile X Syndrome Mutants
- PMID: 30135642
- PMCID: PMC6092503
- DOI: 10.3389/fnmol.2018.00242
Stress Odorant Sensory Response Dysfunction in Drosophila Fragile X Syndrome Mutants
Abstract
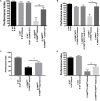
Sensory processing dysfunction (SPD) is present in most patients with intellectual disability (ID) and autism spectrum disorder (ASD). Silencing expression of the Fragile X mental retardation 1 (FMR1) gene leads to Fragile X syndrome (FXS), the most common single gene cause of ID and ASD. Drosophila have a highly conserved FMR1 ortholog, dfmr1. dfmr1 mutants display cognitive and social defects reminiscent of symptoms seen in individuals with FXS. We utilized a robust behavioral assay for sensory processing of the Drosophila stress odorant (dSO) to gain a better understanding of the molecular basis of SPD in FXS. Here, we show that dfmr1 mutant flies present significant defects in dSO response. We found that dfmr1 expression in mushroom bodies is required for dSO processing. We also show that cyclic adenosine monophosphate (cAMP) signaling via PKA is activated after exposure to dSO and that several drugs regulating both cAMP and cyclic guanosine monophosphate (cGMP) levels significantly improved defects in dSO processing in dfmr1 mutant flies.
Keywords: Drosophila; Fragile X syndrome; IBMX; avoidance response; cAMP; cGMP; sensory response dysfunction.
Figures




References
-
- Androschuk A. (2016). Cyclic Adenosine Monophosphate (cAMP) and Fragile X Mental Retardation Protein (FMRP) Mediate Avoidance Behaviour in Drosophila. Master thesis, University of Alberta, Edmonton, AB.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

