Interplay Between Age and Neuroinflammation in Multiple Sclerosis: Effects on Motor and Cognitive Functions
- PMID: 30135651
- PMCID: PMC6092506
- DOI: 10.3389/fnagi.2018.00238
Interplay Between Age and Neuroinflammation in Multiple Sclerosis: Effects on Motor and Cognitive Functions
Abstract
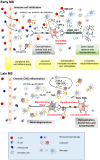
Aging is one of the main risk factors for the development of many neurodegenerative diseases. Emerging evidence has acknowledged neuroinflammation as potential trigger of the functional changes occurring during normal and pathological aging. Two main determinants have been recognized to cogently contribute to neuroinflammation in the aging brain, i.e., the systemic chronic low-grade inflammation and the decline in the regulation of adaptive and innate immune systems (immunosenescence, ISC). The persistence of the inflammatory status in the brain in turn may cause synaptopathy and synaptic plasticity impairments that underlie both motor and cognitive dysfunctions. Interestingly, such inflammation-dependent synaptic dysfunctions have been recently involved in the pathophysiology of multiple sclerosis (MS). MS is an autoimmune neurodegenerative disease, typically affecting young adults that cause an early and progressive deterioration of both cognitive and motor functions. Of note, recent controlled studies have clearly shown that age at onset modifies prognosis and exerts a significant effect on presenting phenotype, suggesting that aging is a significant factor associated to the clinical course of MS. Moreover, some lines of evidence point to the different impact of age on motor disability and cognitive deficits, being the former most affected than the latter. The precise contribution of aging-related factors to MS neurological disability and the underlying molecular and cellular mechanisms are still unclear. In the present review article, we first emphasize the importance of the neuroinflammatory dependent mechanisms, such as synaptopathy and synaptic plasticity impairments, suggesting their potential exacerbation or acceleration with advancing age in the MS disease. Lastly, we provide an overview of clinical and experimental studies highlighting the different impact of age on motor disability and cognitive decline in MS, raising challenging questions on the putative age-related mechanisms involved.
Keywords: aging; cognition; experimental autoimmune encephalomyelitis; multiple sclerosis; neurodegeneration; neuroinflammation; synaptic plasticity; synaptopathy.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

