Associations between fetal size, sex and placental angiogenesis in the pig
- PMID: 30137229
- PMCID: PMC6335214
- DOI: 10.1093/biolre/ioy184
Associations between fetal size, sex and placental angiogenesis in the pig
Abstract
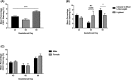
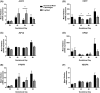
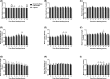
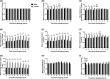
Inadequate fetal growth cannot be remedied postnatally, leading to severe consequences for neonatal and adult development. It is hypothesized that growth restriction occurs due to inadequate placental vascularization. This study investigated the relationship between porcine fetal size, sex, and placental angiogenesis at multiple gestational days (GD). Placental samples supplying the lightest and closest to mean litter weight (CTMLW), male and female Large White X Landrace fetuses were obtained at GD30, 45, 60, and 90. Immunohistochemistry revealed increased chorioallantoic membrane CD31 staining in placentas supplying the lightest compared to those supplying the CTMLW fetuses at GD60. At GD90, placentas supplying the lightest fetuses had decreased CD31 staining in the chorioallantoic membrane compared to those supplying the CTMLW fetuses. The mRNA expression of six candidate genes with central roles at the feto-maternal interface increased with advancing gestation. At GD60, ACP5 expression was increased in placentas supplying the lightest compared to the CTMLW fetuses. At GD45, CD31 expression was decreased in placentas supplying the lightest compared to the CTMLW fetuses. In contrast, CD31 expression was increased in placentas supplying the lightest compared the CTMLW fetuses at GD60. In vitro endothelial cell branching assays demonstrated that placentas supplying the lightest and male fetuses impaired endothelial cell branching compared to placentas from the CTMLW (GD45 and 60) and female fetuses (GD60), respectively. This study has highlighted that placentas supplying the lightest and male fetuses have impaired angiogenesis. Importantly, the relationship between fetal size, sex, and placental vascularity is dynamic and dependent upon the GD investigated.
Figures


Similar articles
-
Novel relationships between porcine fetal size, sex, and endometrial angiogenesis†.Biol Reprod. 2019 Jul 1;101(1):112-125. doi: 10.1093/biolre/ioz068. Biol Reprod. 2019. PMID: 31093645
-
Associations between fetal size, sex and both proliferation and apoptosis at the porcine feto-maternal interface.Placenta. 2018 Oct;70:15-24. doi: 10.1016/j.placenta.2018.08.006. Epub 2018 Aug 27. Placenta. 2018. PMID: 30316322 Free PMC article.
-
Association of foetal size and sex with porcine foeto-maternal interface integrin expression.Reproduction. 2019 Apr 1;157(4):317-328. doi: 10.1530/REP-18-0520. Reproduction. 2019. PMID: 30650060 Free PMC article.
-
Animal models of placental angiogenesis.Placenta. 2005 Nov;26(10):689-708. doi: 10.1016/j.placenta.2004.11.010. Placenta. 2005. PMID: 16226119 Review.
-
Uteroplacental vascular development and placental function: an update.Int J Dev Biol. 2010;54(2-3):355-66. doi: 10.1387/ijdb.082799lr. Int J Dev Biol. 2010. PMID: 19924632 Review.
Cited by
-
Placental Transcriptome Analysis in Connection with Low Litter Birth Weight Phenotype (LBWP) Sows.Genes (Basel). 2024 May 28;15(6):703. doi: 10.3390/genes15060703. Genes (Basel). 2024. PMID: 38927639 Free PMC article.
-
Gestational heat stress alters skeletal muscle gene expression profiles and vascularity in fetal pigs in a sexually dimorphic manner.J Anim Sci Biotechnol. 2022 Jul 15;13(1):76. doi: 10.1186/s40104-022-00730-2. J Anim Sci Biotechnol. 2022. PMID: 35836286 Free PMC article.
-
Sex-bias metabolism of fetal organs, and their relationship to the regulation of fetal brain-placental axis.Metabolomics. 2024 Nov 4;20(6):126. doi: 10.1007/s11306-024-02189-w. Metabolomics. 2024. PMID: 39495316
-
Associations between maternal vitamin D status and porcine litter characteristics throughout gestation.J Anim Sci Biotechnol. 2022 Sep 20;13(1):106. doi: 10.1186/s40104-022-00760-w. J Anim Sci Biotechnol. 2022. PMID: 36123748 Free PMC article.
-
Male fetal sex affects uteroplacental angiogenesis in growth restriction mouse model†.Biol Reprod. 2021 Apr 1;104(4):924-934. doi: 10.1093/biolre/ioab006. Biol Reprod. 2021. PMID: 33459759 Free PMC article.
References
-
- Keys JL, King GJ, Kennedy TG. Increased uterine vascular permeability at the time of embryonic attachment in the pig. Biol Reprod 1986; 34:405–411. - PubMed
-
- Vonnahme KA, Wilson ME, Ford SP. Relationship between placental vascular endothelial growth factor expression and placental/endometrial vascularity in the pig. Biol Reprod 2001; 64:1821–1825. - PubMed
-
- Reynolds LP, Ferrell CL, Robertson DA, Ford SP. Metabolism of the gravid uterus, foetus and utero-placenta at several stages of gestation in cows. J Agric Sci 1986; 106:437–444.
-
- Reynolds LP, Ferrell CL. Transplacental clearance and blood flows of bovine gravid uterus at several stages of gestation. Am J Physiol 1987; 253:735–739. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

