Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes
- PMID: 30137266
- PMCID: PMC6405412
- DOI: 10.1210/er.2018-00089
Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes
Abstract
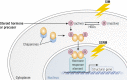
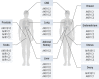
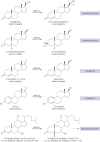
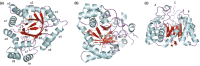
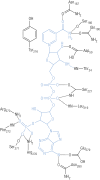
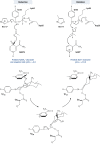
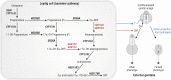
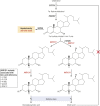
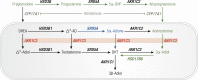
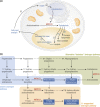
Aldo-keto reductases (AKRs) are monomeric NAD(P)(H)-dependent oxidoreductases that play pivotal roles in the biosynthesis and metabolism of steroids in humans. AKR1C enzymes acting as 3-ketosteroid, 17-ketosteroid, and 20-ketosteroid reductases are involved in the prereceptor regulation of ligands for the androgen, estrogen, and progesterone receptors and are considered drug targets to treat steroid hormone-dependent malignancies and endocrine disorders. In contrast, AKR1D1 is the only known steroid 5β-reductase and is essential for bile-acid biosynthesis, the generation of ligands for the farnesoid X receptor, and the 5β-dihydrosteroids that have their own biological activity. In this review we discuss the crystal structures of these AKRs, their kinetic and catalytic mechanisms, AKR genomics (gene expression, splice variants, polymorphic variants, and inherited genetic deficiencies), distribution in steroid target tissues, roles in steroid hormone action and disease, and inhibitor design.
Copyright © 2019 Endocrine Society.
Figures



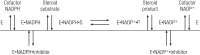

References
-
- Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242(4878):583–585. - PubMed
-
- Penning TM. Hydroxysteroid dehydrogenases and pre-receptor regulation of steroid hormone action. Hum Reprod Update. 2003;9(3):193–205. - PubMed
-
- Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

