MeCP2 isoform e1 mutant mice recapitulate motor and metabolic phenotypes of Rett syndrome
- PMID: 30137367
- PMCID: PMC6240741
- DOI: 10.1093/hmg/ddy301
MeCP2 isoform e1 mutant mice recapitulate motor and metabolic phenotypes of Rett syndrome
Abstract
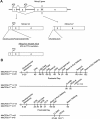
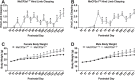
Mutations in the X-linked gene MECP2 cause the majority of Rett syndrome (RTT) cases. Two differentially spliced isoforms of exons 1 and 2 (MeCP2-e1 and MeCP2-e2) contribute to the diverse functions of MeCP2, but only mutations in exon 1, not exon 2, are observed in RTT. We previously described an isoform-specific MeCP2-e1-deficient male mouse model of a human RTT mutation that lacks MeCP2-e1 while preserving expression of MeCP2-e2. However, RTT patients are heterozygous females that exhibit delayed and progressive symptom onset beginning in late infancy, including neurologic as well as metabolic, immune, respiratory and gastrointestinal phenotypes. Consequently, we conducted a longitudinal assessment of symptom development in MeCP2-e1 mutant females and males. A delayed and progressive onset of motor impairments was observed in both female and male MeCP2-e1 mutant mice, including hind limb clasping and motor deficits in gait and balance. Because these motor impairments were significantly impacted by age-dependent increases in body weight, we also investigated metabolic phenotypes at an early stage of disease progression. Both male and female MeCP2-e1 mutants exhibited significantly increased body fat compared to sex-matched wild-type littermates prior to weight differences. Mecp2e1-/y males exhibited significant metabolic phenotypes of hypoactivity, decreased energy expenditure, increased respiratory exchange ratio, but decreased food intake compared to wild-type. Untargeted analysis of lipid metabolites demonstrated a distinguishable profile in MeCP2-e1 female mutant liver characterized by increased triglycerides. Together, these results demonstrate that MeCP2-e1 mutation in mice of both sexes recapitulates early and progressive metabolic and motor phenotypes of human RTT.
Figures







References
-
- Mnatzakanian G.N., Lohi H., Munteanu I., Alfred S.E., Yamada T., MacLeod P.J.M., Jones J.R., Scherer S.W., Schanen N.C., Friez M.J. et al. (2004) A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat. Genet., 36, 339–341. - PubMed
-
- Gianakopoulos P.J., Zhang Y., Pencea N., Orlic-Milacic M., Mittal K., Windpassinger C., White S.J., Kroisel P.M., Chow E.W.C., Saunders C.J. et al. (2012) Mutations in MECP2 exon 1 in classical Rett patients disrupt MECP2_e1 transcription, but not transcription of MECP2_e2. Am. J. Med. Genet. Part B Neuropsychiatr. Genet., 159B, 210–216. - PubMed
-
- Saunders C.J., Minassian B.E., Chow E.W.C., Zhao W. and Vincent J.B. (2009) Novel exon 1 mutations in MECP2 implicate isoform MeCP2-e1 in classical Rett syndrome. Am. J. Med. Genet. Part A, 149, 1019–1023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

