Controlling Thermal Expansion Behaviors of Fence-Like Metal-Organic Frameworks by Varying/Mixing Metal Ions
- PMID: 30137745
- PMCID: PMC6066979
- DOI: 10.3389/fchem.2018.00306
Controlling Thermal Expansion Behaviors of Fence-Like Metal-Organic Frameworks by Varying/Mixing Metal Ions
Abstract
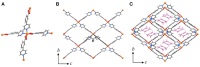
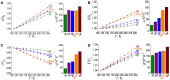
Solvothermal reactions of 3-(4-pyridyl)-benzoic acid (Hpba) with a series of transition metal ions yielded isostructral metal-organic frameworks [M(pba)2]·2DMA (MCF-52; M = Ni2+, Co2+, Zn2+, Cd2+, or mixed Zn2+/Cd2+; DMA = N,N-dimethylacetamide) possessing two-dimensional fence-like coordination networks based on mononuclear 4-connected metal nodes and 2-connected organic ligands. Variable-temperature single-crystal X-ray diffraction studies of these materials revealed huge positive and negative thermal expansions with |α| > 150 × 10-6 K-1, in which the larger metal ions give the larger thermal expansion coefficients, because the increased space not only enhance the ligand vibrational motion and hinged-fence effect, but also allow larger changes of steric hindrance between the layers. In addition, the solid-solution crystal with mixed metal ions further validates the abundant thermal expansion mechanisms of these metal-organic layers.
Keywords: flexibility; metal ion radius; metal-organic framework; porous coordination polymers; pyridyl-carboxylate; solid solution; structure-property relationship; thermal expansion.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

