Improved Survival for Children and Young Adults With T-Lineage Acute Lymphoblastic Leukemia: Results From the Children's Oncology Group AALL0434 Methotrexate Randomization
- PMID: 30138085
- PMCID: PMC6366301
- DOI: 10.1200/JCO.2018.77.7250
Improved Survival for Children and Young Adults With T-Lineage Acute Lymphoblastic Leukemia: Results From the Children's Oncology Group AALL0434 Methotrexate Randomization
Erratum in
-
Errata.J Clin Oncol. 2019 Mar 20;37(9):761. doi: 10.1200/JCO.19.00295. J Clin Oncol. 2019. PMID: 30875487 Free PMC article. No abstract available.
Abstract
Purpose: Early intensification with methotrexate (MTX) is a key component of acute lymphoblastic leukemia (ALL) therapy. Two different approaches to MTX intensification exist but had not been compared in T-cell ALL (T-ALL): the Children's Oncology Group (COG) escalating dose intravenous MTX without leucovorin rescue plus pegaspargase escalating dose, Capizzi-style, intravenous MTX (C-MTX) regimen and the Berlin-Frankfurt-Muenster (BFM) high-dose intravenous MTX (HDMTX) plus leucovorin rescue regimen.
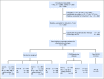
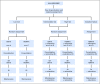
Patients and methods: COG AALL0434 included a 2 × 2 randomization that compared the COG-augmented BFM (ABFM) regimen with either C-MTX or HDMTX during the 8-week interim maintenance phase. All patients with T-ALL, except for those with low-risk features, received prophylactic (12 Gy) or therapeutic (18 Gy for CNS3) cranial irradiation during either the consolidation (C-MTX; second month of therapy) or delayed intensification (HDMTX; seventh month of therapy) phase.
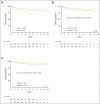
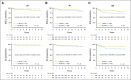
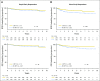
Results: AALL0434 accrued 1,895 patients from 2007 to 2014. The 5-year event-free survival and overall survival rates for all eligible, evaluable patients with T-ALL were 83.8% (95% CI, 81.2% to 86.4%) and 89.5% (95% CI, 87.4% to 91.7%), respectively. The 1,031 patients with T-ALL but without CNS3 disease or testicular leukemia were randomly assigned to receive ABFM with C-MTX (n = 519) or HDMTX (n = 512). The estimated 5-year disease-free survival ( P = .005) and overall survival ( P = .04) rates were 91.5% (95% CI, 88.1% to 94.8%) and 93.7% (95% CI, 90.8% to 96.6%) for C-MTX and 85.3% (95% CI, 81.0%-89.5%) and 89.4% (95% CI, 85.7%-93.2%) for HDMTX. Patients assigned to C-MTX had 32 relapses, six with CNS involvement, whereas those assigned to HDMTX had 59 relapses, 23 with CNS involvement.
Conclusion: AALL0434 established that ABFM with C-MTX was superior to ABFM plus HDMTX for T-ALL in approximately 90% of patients who received CRT, with later timing for those receiving HDMTX.
Trial registration: ClinicalTrials.gov NCT00408005.
Figures

References
-
- Steinherz PG, Gaynon PS, Breneman JC, et al. : Treatment of patients with acute lymphoblastic leukemia with bulky extramedullary disease and T-cell phenotype or other poor prognostic features: Randomized controlled trial from the Children’s Cancer Group. Cancer 82:600-612, 1998 - PubMed
-
- Uckun FM, Sensel MG, Sun L, et al. : Biology and treatment of childhood T-lineage acute lymphoblastic leukemia. Blood 91:735-746, 1998 - PubMed
-
- Schrappe M, Valsecchi MG, Bartram CR, et al. : Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood 118:2077-2084, 2011 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

