The cat as a naturally occurring model of renal interstitial fibrosis: Characterisation of primary feline proximal tubular epithelial cells and comparative pro-fibrotic effects of TGF-β1
- PMID: 30138414
- PMCID: PMC6107233
- DOI: 10.1371/journal.pone.0202577
The cat as a naturally occurring model of renal interstitial fibrosis: Characterisation of primary feline proximal tubular epithelial cells and comparative pro-fibrotic effects of TGF-β1
Abstract
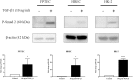
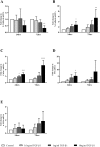
Chronic kidney disease (CKD) is common in both geriatric cats and aging humans, and is pathologically characterised by chronic tubulointerstitial inflammation and fibrosis in both species. Cats with CKD may represent a spontaneously occurring, non-rodent animal model of human disease, however little is known of feline renal cell biology. In other species, TGF-β1 signalling in the proximal tubular epithelium is thought to play a key role in the initiation and progression of renal fibrosis. In this study, we first aimed to isolate and characterise feline proximal tubular epithelial cells (FPTEC), comparing them to human primary renal epithelial cells (HREC) and the human proximal tubular cell line HK-2. Secondly, we aimed to examine and compare the effect of human recombinant TGF-β1 on cell proliferation, pro-apoptotic signalling and genes associated with epithelial-to-mesenchymal transition (EMT) in feline and human renal epithelial cells. FPTEC were successfully isolated from cadaverous feline renal tissue, and demonstrated a marker protein expression profile identical to that of HREC and HK-2. Exposure to TGF-β1 (0-10 ng/ml) induced a concentration-dependent loss of epithelial morphology and alterations in gene expression consistent with the occurrence of partial EMT in all cell types. This was associated with transcription of downstream pro-fibrotic mediators, growth arrest in FPTEC and HREC (but not HK-2), and increased apoptotic signalling at high concentrations of TGF- β1. These effects were inhibited by the ALK5 (TGF-β1RI) antagonist SB431542 (5 μM), suggesting they are mediated via the ALK5/TGF-β1RII receptor complex. Taken together, these results suggest that TGF-β1 may be involved in epithelial cell dedifferentiation, growth arrest and apoptosis in feline CKD as in human disease, and that cats may be a useful, naturally occurring model of human CKD.
Conflict of interest statement
JE and HS are members of the International Renal Interest Society, which is sponsored by Elanco Ltd. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of the paper. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures








Similar articles
-
Investigation of the transforming growth factor-beta 1 signalling pathway as a possible link between hyperphosphataemia and renal fibrosis in feline chronic kidney disease.Vet J. 2021 Jan;267:105582. doi: 10.1016/j.tvjl.2020.105582. Epub 2020 Nov 28. Vet J. 2021. PMID: 33375963 Free PMC article.
-
Characterisation of feline renal cortical fibroblast cultures and their transcriptional response to transforming growth factor β1.BMC Vet Res. 2018 Mar 9;14(1):76. doi: 10.1186/s12917-018-1387-2. BMC Vet Res. 2018. PMID: 29523136 Free PMC article.
-
Pterostilbene alleviates fructose-induced renal fibrosis by suppressing TGF-β1/TGF-β type I receptor/Smads signaling in proximal tubular epithelial cells.Eur J Pharmacol. 2019 Jan 5;842:70-78. doi: 10.1016/j.ejphar.2018.10.008. Epub 2018 Oct 16. Eur J Pharmacol. 2019. PMID: 30336139
-
TGF-β1-p53 cooperativity regulates a profibrotic genomic program in the kidney: molecular mechanisms and clinical implications.FASEB J. 2019 Oct;33(10):10596-10606. doi: 10.1096/fj.201900943R. Epub 2019 Jul 6. FASEB J. 2019. PMID: 31284746 Free PMC article. Review.
-
Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities.Clin Sci (Lond). 2021 Jan 29;135(2):275-303. doi: 10.1042/CS20201213. Clin Sci (Lond). 2021. PMID: 33480423 Review.
Cited by
-
Vitamin D Metabolism and Its Role in Mineral and Bone Disorders in Chronic Kidney Disease in Humans, Dogs and Cats.Metabolites. 2020 Dec 4;10(12):499. doi: 10.3390/metabo10120499. Metabolites. 2020. PMID: 33291777 Free PMC article. Review.
-
Development of a Model of Tubulointerstitial Fibrosis Using Transient Unilateral Renal Ischemia and Delayed Contralateral Nephrectomy in Domesticated Cats.Comp Med. 2024 Aug 1;74(4):274-283. doi: 10.30802/AALAS-CM-24-000025. Epub 2024 Jun 20. Comp Med. 2024. PMID: 38902012 Free PMC article.
-
The Multicomponent, Multitarget Therapy SUC in Cats with Chronic Kidney Disease: A Multicenter, Prospective, Observational, Nonrandomized Cohort Study.Complement Med Res. 2020;27(3):163-173. doi: 10.1159/000506698. Epub 2020 Mar 26. Complement Med Res. 2020. PMID: 32213769 Free PMC article.
-
Chemokine Therapy in Cats With Experimental Renal Fibrosis and in a Kidney Disease Pilot Study.Front Vet Sci. 2021 Mar 4;8:646087. doi: 10.3389/fvets.2021.646087. eCollection 2021. Front Vet Sci. 2021. PMID: 33748219 Free PMC article.
-
Prostacyclin analog beraprost sodium efficacy in primary glomerular disease or nephrosclerosis: Analysis of the Japanese subgroup in CASSIOPEIR study.Ther Apher Dial. 2021 Oct;25(5):551-564. doi: 10.1111/1744-9987.13616. Epub 2021 Feb 21. Ther Apher Dial. 2021. PMID: 33340238 Free PMC article. Clinical Trial.
References
-
- Marino CL, Lascelles BD, Vaden SL, Gruen ME, Marks SL. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. Journal of feline medicine and surgery. 2014;16(6):465–72. 10.1177/1098612X13511446 . - DOI - PMC - PubMed
-
- DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). Journal of the American Veterinary Medical Association. 1987;190(9):1196–202. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

