Manchette-acrosome disorders and testicular efficiency decline observed in hypercholesterolemic rabbits are recovered with olive oil enriched diet
- PMID: 30138421
- PMCID: PMC6107225
- DOI: 10.1371/journal.pone.0202748
Manchette-acrosome disorders and testicular efficiency decline observed in hypercholesterolemic rabbits are recovered with olive oil enriched diet
Abstract
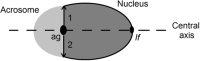
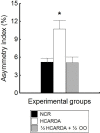
High-fat diet is associated with hypercholesterolemia and seminal alterations in White New Zealand rabbits. We have previously reported disorders in the development of the manchette-acrosome complex during spermiogenesis and decreased testicular efficiency in hypercholesterolemic rabbits. On the other hand, olive oil incorporated into the diet improves cholesterolemia and semen parameters affected in hypercholesterolemic rabbits. In this paper, we report the recovery-with the addition of olive oil to diet-from the sub-cellular mechanisms involved in the shaping of the sperm cell and testicular efficiency altered in hypercholesterolemic rabbits. Using morphological (structural, ultra-structural and immuno-fluorescence techniques) and cell biology techniques, a reorganization of the manchette and related structures was observed when olive oil was added to the high-fat diet. Specifically, actin filaments, microtubules and lipid rafts-abnormally distributed in hypercholesterolemic rabbits-were recovered with dietary olive oil supplementation. The causes of the decline in sperm count were studied in the previous report and here in more detail. These were attributed to the decrease in the efficiency index and also to the increase in the apoptotic percentage in testis from animals under the high-fat diet. Surprisingly, the addition of olive oil to the diet avoided the sub-cellular, efficiency and apoptosis changes observed in hypercholesterolemic rabbits. This paper reports the positive effects of the olive oil addition to the diet in the recovery of testicular efficiency and normal sperm shaping, mechanisms altered by hypercholesterolemia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








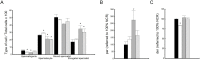
Similar articles
-
Exploring the impact of lipid stress on sperm cytoskeleton: insights and prospects.Nat Rev Urol. 2025 May;22(5):294-312. doi: 10.1038/s41585-024-00952-1. Epub 2024 Nov 11. Nat Rev Urol. 2025. PMID: 39528754 Review.
-
Manchette-acrosome disorders during spermiogenesis and low efficiency of seminiferous tubules in hypercholesterolemic rabbit model.PLoS One. 2017 Feb 27;12(2):e0172994. doi: 10.1371/journal.pone.0172994. eCollection 2017. PLoS One. 2017. PMID: 28241054 Free PMC article.
-
Semen quality and sperm function loss by hypercholesterolemic diet was recovered by addition of olive oil to diet in rabbit.PLoS One. 2013;8(1):e52386. doi: 10.1371/journal.pone.0052386. Epub 2013 Jan 11. PLoS One. 2013. PMID: 23326331 Free PMC article.
-
Extra-virgin olive oil ameliorates high-fat diet-induced seminal and testicular disorders by modulating the cholesterol pathway.Andrology. 2023 Sep;11(6):1203-1217. doi: 10.1111/andr.13398. Epub 2023 Feb 11. Andrology. 2023. PMID: 36695747
-
Function of manchette and intra-manchette transport in spermatogenesis and male fertility.Cell Commun Signal. 2025 May 29;23(1):250. doi: 10.1186/s12964-025-02213-z. Cell Commun Signal. 2025. PMID: 40442757 Free PMC article. Review.
Cited by
-
A chronic high-fat diet causes sperm head alterations in C57BL/6J mice.Heliyon. 2019 Nov 21;5(11):e02868. doi: 10.1016/j.heliyon.2019.e02868. eCollection 2019 Nov. Heliyon. 2019. PMID: 31844747 Free PMC article.
-
Bioactive compounds as potential alternative treatments to prevent cancer therapy-induced male infertility.Front Endocrinol (Lausanne). 2024 Jan 18;14:1293780. doi: 10.3389/fendo.2023.1293780. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38303979 Free PMC article. Review.
-
Is a Ketogenic Diet Superior to a High-Fat, High-Cholesterol Diet Regarding Testicular Function and Spermatogenesis?Front Nutr. 2022 Feb 10;9:805794. doi: 10.3389/fnut.2022.805794. eCollection 2022. Front Nutr. 2022. PMID: 35223950 Free PMC article.
-
Exploring the impact of lipid stress on sperm cytoskeleton: insights and prospects.Nat Rev Urol. 2025 May;22(5):294-312. doi: 10.1038/s41585-024-00952-1. Epub 2024 Nov 11. Nat Rev Urol. 2025. PMID: 39528754 Review.
-
Dietary Cholesterol and Lipid Overload: Impact on Male Fertility.Oxid Med Cell Longev. 2019 Dec 6;2019:4521786. doi: 10.1155/2019/4521786. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885793 Free PMC article. Review.
References
-
- Marchiani S, Vignozzi L, Filippi S, Gurrieri B, Comeglio P, Morelli A, et al. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: Relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol Cell Endocrinol. 2015. February;401:12–24. 10.1016/j.mce.2014.11.005 - DOI - PubMed
-
- Boarelli PV, Monclus MA, Saez Lancellotti T, Cabrillana ME, Simón L, Funes AK, et al. Simultaneous Study of Cholesterol and GM1 Ganglioside by Specific Probes: Lipid Distribution during Maturation, Capacitation and the Acrosome Reaction. J Cytol Histol. 2016. April;07:412 10.4172/2157-7099.1000412 - DOI
-
- Richard C, Couture P, Desroches S, Benjannet S, Seidah NG, Lichtenstein AH, et al. Effect of the Mediterranean diet with and without weight loss on surrogate markers of cholesterol homeostasis in men with the metabolic syndrome. Br J Nutr. 2012. March;107(5):705–11. 10.1017/S0007114511003436 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

