Carbohydrate supplementation of human milk to promote growth in preterm infants
- PMID: 30138549
- PMCID: PMC6513426
- DOI: 10.1002/14651858.CD000280.pub2
Carbohydrate supplementation of human milk to promote growth in preterm infants
Update in
-
Carbohydrate supplementation of human milk to promote growth in preterm infants.Cochrane Database Syst Rev. 2020 Sep 8;9(9):CD000280. doi: 10.1002/14651858.CD000280.pub3. Cochrane Database Syst Rev. 2020. PMID: 32898300 Free PMC article.
Abstract
Background: Preterm infants are born with low glycogen stores and require higher glucose intake to match fetal accretion rates. In spite of the myriad benefits of breast milk for preterm infants, it may not adequately meet the needs of these rapidly growing infants. Supplementing human milk with carbohydrates may help. However, there is a paucity of data on assessment of benefits or harms of carbohydrate supplementation of human milk to promote growth in preterm infants. This is a 2018 update of a Cochrane Review first published in 1999.
Objectives: To determine whether human milk supplemented with carbohydrate compared with unsupplemented human milk fed to preterm infants improves growth, body composition, and cardio-metabolic and neurodevelopmental outcomes without significant adverse effects.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8), MEDLINE via PubMed (1966 to 21 February 2018), Embase (1980 to 21 February 2018), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 21 February 2018). We also searched clinical trials databases, conference proceedings, and reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi-randomised trials.
Selection criteria: Published and unpublished controlled trials were eligible if they used random or quasi-random methods to allocate preterm infants in hospital fed human milk to supplementation or no supplementation with additional carbohydrate.
Data collection and analysis: Two review authors independently abstracted data and assessed trial quality and the quality of evidence at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method. We planned to perform meta-analyses using risk ratios (RRs) for dichotomous data and mean differences (MDs) for continuous data, with their respective 95% confidence intervals (CIs). We planned to use a fixed-effect model and to explore potential causes of heterogeneity via sensitivity analyses. We contacted study authors for additional information.
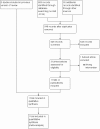
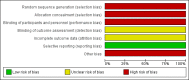
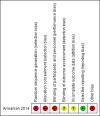
Main results: One unblinded, quasi-randomised controlled trial (RCT) assessing effects of carbohydrate supplementation of human milk in the form of a prebiotic in 75 preterm infants was eligible for inclusion in this review. We identified two publications of the same trial, which reported different methods regarding blinding and randomisation. Study authors confirmed that these publications pertain to the same trial, but they have not yet clarified which method is correct. We were unable to reproduce analyses from the data presented. At 30 days of age, the mean weight of preterm infants in the trial was greater in the prebiotic carbohydrate-supplemented group than in the unsupplemented group (MD 160.4 grams, 95% CI 12.4 to 308.4 grams; one RCT, N = 75; very low-quality evidence). We found no evidence of a clear difference in risk of feeding intolerance (RR 0.64, 95% CI 0.36 to 1.15; one RCT, N = 75 infants; very low-quality evidence) or necrotising enterocolitis (NEC) (RR 0.2, 95% CI 0.02 to 1.3; one RCT, N = 75 infants; very low-quality evidence) between the prebiotic-supplemented group and the unsupplemented group. Duration of hospital stay was shorter in the prebiotic group than in the control group at a median (range) of 16 (9 to 45) days (95% CI 15.34 to 24.09) and 25 (11 to 80) days (95% CI 25.52 to 34.39), respectively. No other data were available for assessing effects of carbohydrate supplementation on short- and long-term growth, body mass index, body composition, and neurodevelopmental or cardio-metabolic outcomes.
Authors' conclusions: We found insufficient evidence on the short- and long-term effects of carbohydrate supplementation of human milk in preterm infants. The only trial included in this review presented very low-quality evidence, and study authors provided uncertain information about study methods and analysis. The evidence may be limited in its applicability because researchers included a small sample of preterm infants from a single centre. However, the outcomes assessed are common to all preterm infants, and this trial demonstrates the feasibility of prebiotic carbohydrate supplementation in upper-middle-income countries. Future trials should assess the safety and efficacy of different types and concentrations of carbohydrate supplementation for preterm infants fed human milk. Although prebiotic carbohydrate supplementation in preterm infants is currently a topic of active research, we do not envisage that further trials of digestible carbohydrates will be conducted, as this is currently done as a component of multi-nutrient human milk fortification. Hence we do not plan to publish any further updates of this review.
Conflict of interest statement
Emma Amissah: none known.
Julie Brown: none known.
Jane Harding: none known.
Figures



Update of
-
Carbohydrate supplementation of human milk to promote growth in preterm infants.Cochrane Database Syst Rev. 2000;(2):CD000280. doi: 10.1002/14651858.CD000280. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2018 Aug 23;8:CD000280. doi: 10.1002/14651858.CD000280.pub2. PMID: 10796191 Updated.
References
References to studies included in this review
Armanian 2014 {published data only}
-
- Armanian AM, Sadeghina A, Hoseinzadeh M, Mirlohi M, Feizi A, Salehimehr N, et al. The effect of neutral oligosaccharides on reducing the incidence of necrotizing enterocolitis in preterm infants: a randomized clinical trial. International Journal of Preventive Medicine 2014;5(11):1387‐95. [PUBMED: 25538834] - PMC - PubMed
-
- Armanian AM, Sadeghnia A, Hoseinzadeh M, Mirlohi M, Feizi A, Salehimehr N, et al. The effect of neutral oligosaccharides on fecal microbiota in premature infants fed exclusively with breast milk: a randomized clinical trial. Journal of Research in Pharmacy Practice 2016;5(1):27‐34. [DOI: 10.4103/2279-042X.176558; PUBMED: 26985433] - DOI - PMC - PubMed
References to studies excluded from this review
Nandhini 2016 {published data only}
-
- Nandhini LP, Biswal N, Adhisivam B, Mandal J, Bhat BV, Mathai B. Synbiotics for decreasing incidence of necrotizing enterocolitis among preterm neonates ‐ a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(5):821‐5. [DOI: 10.3109/14767058.2015.1019854; PUBMED: 25754214] - DOI - PubMed
Additional references
Armanian 2016
-
- Armanian AM, Sadeghnia A, Hoseinzadeh M, Mirlohi M, Feizi A, Salehimehr N, et al. The effect of neutral oligosaccharides on fecal microbiota in premature infants fed exclusively with breast milk: a randomized clinical trial. Journal of Research in Pharmacy Practice 2016;5(1):27‐34. [DOI: 10.4103/2279-042X.176558; PUBMED: 26985433] - DOI - PMC - PubMed
Ayede 2011
Ballard 2013
Belfort 2016
-
- Belfort MB, Anderson PJ, Nowak VA, Lee KJ, Molesworth C, Thompson DK, et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7‐year longitudinal study in infants born at less than 30 weeks' gestation. Journal of Pediatrics 2016;177:133‐9.e1. [DOI: 10.1016/j.jpeds.2016.06.045; PUBMED: 27480198] - DOI - PMC - PubMed
Blackburn 2017
-
- Blackburn S. Maternal, Fetal, & Neonatal Physiology ‐ E‐book: A Clinical Perspective. St. Louis, Missouri: Elsevier Health Sciences, 2017.
Blank 2012
Bode 2012
Brown 2014
Brown 2016
Cowett 2012
-
- Cowett RM. Principles of Perinatal‐Neonatal Metabolism. 2nd Edition. New York: Springer Science & Business Media, 2012 (reprint of 1998 edition).
Duggan 2008
-
- Duggan C, Watkins JB, Walker WA. Nutrition in Pediatrics: Basic Science, Clinical Applications. Hamilton, Ontario: BC Decker Inc, 2008.
Duijts 2010
Elzouki 2012
-
- Elzouki AY, Harfi HA, Nazer H, Stapleton FB, Oh W, Whitley RJ (editors). Textbook of Clinical Pediatrics. 2nd Edition. New York: Springer Science & Business Media, 2012.
Erasmus 2002
Fanaroff 2012
-
- Fanaroff JM, Fanaroff AA. Klaus and Fanaroff's Care of the High‐Risk Neonate E‐Book. 6th Edition. St. Louis, Missouri: Elsevier Health Sciences, 2012.
Fenton 2013
Gabrielli 2011
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 01 September 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gregory 2005
Hay 2008
Hay 2017
Heine 2017
-
- Heine RG, AlRefaee F, Bachina P, Leon JC, Geng L, Gong S, et al. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children – common misconceptions revisited. World Allergy Organization Journal 2017;10(1):41. [DOI: 10.1186/s40413-017-0173-0; PUBMED: 29270244] - DOI - PMC - PubMed
Hey 2014
Higgins 2017
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from https://training.cochrane.org/handbook.
Jantscher‐Krenn 2012
-
- Jantscher‐Krenn E, Bode L. Human milk oligosaccharides and their potential benefits for the breast‐fed neonate. Minerva Pediatrica 2012;64(1):83‐99. [PUBMED: 22350049] - PubMed
Klein 2002
Kuschel 2000
Mahajan 2017
-
- Mahajan S, Chawla D, Kaur J, Jain S. Macronutrients in breast milk of mothers of preterm infants. Indian Pediatrics 2017;54(8):635‐7. [PUBMED: 28607212] - PubMed
Mangili 2017
Martin 2016
Morrow 2011
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sullivan 2010
-
- Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl‐Kohlendorfer U, et al. An exclusively human milk‐based diet Is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk‐based products. Journal of Pediatrics 2010;156(4):562‐7. [DOI: 10.1016/j.jpeds.2009.10.040; PUBMED: 20036378] - DOI - PubMed
Velaphi 2011
-
- Velaphi S. Nutritional requirements and parenteral nutrition in preterm infants. South African Journal of Clinical Nutrition 2011;24(3):S27‐31.
Vohr 2006
-
- Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, et al. NICHD Neonatal Research Network. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics 2006;118(1):e115‐123. [DOI: 10.1542/peds.2005-2382; PUBMED: 16818526] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

