TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells
- PMID: 30138589
- PMCID: PMC6360533
- DOI: 10.1016/j.neuron.2018.07.033
TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells
Abstract
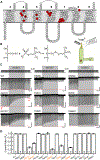
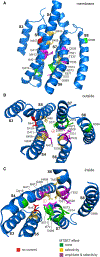
The proteins that form the permeation pathway of mechanosensory transduction channels in inner-ear hair cells have not been definitively identified. Genetic, anatomical, and physiological evidence support a role for transmembrane channel-like protein (TMC) 1 in hair cell sensory transduction, yet the molecular function of TMC proteins remains unclear. Here, we provide biochemical evidence suggesting TMC1 assembles as a dimer, along with structural and sequence analyses suggesting similarity to dimeric TMEM16 channels. To identify the pore region of TMC1, we used cysteine mutagenesis and expressed mutant TMC1 in hair cells of Tmc1/2-null mice. Cysteine-modification reagents rapidly and irreversibly altered permeation properties of mechanosensory transduction. We propose that TMC1 is structurally similar to TMEM16 channels and includes ten transmembrane domains with four domains, S4-S7, that line the channel pore. The data provide compelling evidence that TMC1 is a pore-forming component of sensory transduction channels in auditory and vestibular hair cells.
Keywords: TMC1; TMC2; auditory; balance; hair cell; hearing; mechanosensory transduction; mechanotransduction; sensory transduction; vestibular.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
J.R.H. holds patents on
Figures






Comment in
-
Honing In on TMC as the Hair Cell's Transduction Channel.Neuron. 2018 Aug 22;99(4):628-629. doi: 10.1016/j.neuron.2018.08.013. Neuron. 2018. PMID: 30138584
References
-
- Akabas MH, Stauffer DA, Xu M, and Karlin A (1992). Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science 258, 307–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

