The Nonclinical Safety Profile of GalNAc-conjugated RNAi Therapeutics in Subacute Studies
- PMID: 30139307
- PMCID: PMC6249674
- DOI: 10.1177/0192623318792537
The Nonclinical Safety Profile of GalNAc-conjugated RNAi Therapeutics in Subacute Studies
Abstract
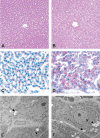
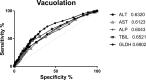
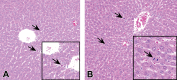
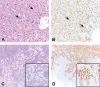
Short interfering RNAs (siRNAs) and antisense oligonucleotides (ASOs) are the most clinically advanced oligonucleotide-based platforms. A number of N-acetylgalactosamine (GalNAc)-conjugated siRNAs (GalNAc-siRNAs), also referred to as RNA interference (RNAi) therapeutics, are currently in various stages of development, though none is yet approved. While the safety of ASOs has been the subject of extensive review, the nonclinical safety profiles of GalNAc-siRNAs have not been reported. With the exception of sequence differences that confer target RNA specificity, GalNAc-siRNAs are largely chemically uniform, containing limited number of phosphorothioate linkages, and 2'-O-methyl and 2'-deoxy-2'-fluoro ribose modifications. Here, we present the outcomes of short-term (3-5 week) rat and monkey weekly repeat-dose toxicology studies of six Enhanced Stabilization Chemistry GalNAc-siRNAs currently in clinical development. In nonclinical studies at supratherapeutic doses, these molecules share similar safety signals, with histologic findings in the organ of pharmacodynamic effect (liver), the organ of elimination (kidney), and the reticuloendothelial system (lymph nodes). The majority of these changes are nonadverse, partially to completely reversible, correlate well with pharmacokinetic parameters and tissue distribution, and often reflect drug accumulation. Furthermore, all GalNAc-siRNAs tested to date have been negative in genotoxicity and safety pharmacology studies.
Keywords: cell(ular) pathology; drug development; liver; monkey pathology; preclinical research and development; preclinical safety assessment/risk management; rat pathology.
Conflict of interest statement
Figures


References
-
- Adityanjee. (1987). Role of electroconvulsive therapy in neuroleptic malignant syndrome (NMS). Acta Psychiatr Scand 76, 603–4. - PubMed
-
- Baenziger J. U., Fiete D. (1980). Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell 22, 611–20. - PubMed
-
- Berman C. L., Barros S. A., Galloway S. M., Kasper P., Oleson F. B., Priestley C. C., Sweder K. S., Schlosser M. J., Sobol Z. (2016). OSWG recommendations for genotoxicity testing of novel oligonucleotide-based therapeutics. Nucleic Acid Ther 26, 73–85. - PubMed
-
- Birmingham A., Anderson E. M., Reynolds A., Ilsley-Tyree D., Leake D., Fedorov Y., Baskerville S., et al. (2006). 3’ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods 3, 199–204. - PubMed
-
- Braasch D. A., Corey D. R. (2001). Locked nucleic acid (LNA): Fine-tuning the recognition of DNA and RNA. Chem Biol 8, 1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

