Study protocol for a controlled trial of an eHealth system utilising patient reported outcome measures for personalised treatment and care: PROMPT-Care 2.0
- PMID: 30139331
- PMCID: PMC6107942
- DOI: 10.1186/s12885-018-4729-3
Study protocol for a controlled trial of an eHealth system utilising patient reported outcome measures for personalised treatment and care: PROMPT-Care 2.0
Abstract
Background: Routine assessment and clinical utilisation of patient-reported outcome (PRO) measures can lead to improved patient outcomes. The PROMPT-Care eHealth system facilitates PRO data capture from cancer patients, data linkage and retrieval to support clinical decisions, patient self-management, and shared care. Pilot testing demonstrated acceptability and feasibility of PROMPT-Care Version 1.0. This study aims to implement PROMPT-Care Version 2.0 and determine its efficacy in reducing emergency department (ED) presentations, and improving chemotherapy delivery and health service referrals, compared to usual care.
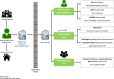
Methods: Groups eligible to participate in the intervention arm of this controlled trial are patients receiving cancer care (including follow-up). PROMPT-Care patients will complete monthly assessments (distress, symptoms, unmet needs) until voluntary withdrawal or death. In Version 1.0, the care team accessed patients' clinical feedback reports in 'real time' to guide their care, and patients received links to support their self-management, tailored to their PRO responses. Version 2.0 was extended to include: i) an additional alert system notifying the care team of ongoing unresolved clinical issues, ii) patient self-management resources, and iii) an auto-populated Treatment Summary and Survivorship Care Plan (SCP). The control population will be patients extracted from hospital databases of the general cancer patient population who were seen at the participating cancer therapy centres during the study period, with a ratio of 1:4 of intervention to control patients. A minimum sample size of 1760 (352 intervention and 1408 control) patients will detect a 14% reduction in the number of ED presentations (primary outcome) in the PROMPT-Care group compared with the control group. Intervention patients will provide feedback on system usability and value of the self-management materials; oncology staff will provide feedback on usefulness of PROMPT-Care reports, response to clinical alerts, impact on routine care, and usefulness of the SCPs; and GPs will provide feedback on the usefulness of the SCPs and attitudes towards shared-care models of survivorship care planning.
Discussion: This study will inform the PROMPT-Care system's impact on healthcare utilisation and utility as an alternative model for ongoing supportive care.
Trial registration: Australian New Zealand Clinical Trials Registry ( ACTRN12616000615482 ) on 12th May 2016 ( www.anzctr.org.au ).
Keywords: Electronic health record; Non-randomized control trial; Patient-centred care; Patient-reported outcomes (PROs); Risk-stratified care; Screening; Self-management; Supportive care; Survivorship care; eHealth.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from the Human Research Ethics Committees of South Western Sydney, and Illawarra Shoalhaven Local Health Districts, with site-specific governance approvals obtained for Liverpool, Campbelltown, Wollongong and Shoalhaven Hospitals.
Participants will be provided with a study pack containing a letter of invitation, participant information sheet and consent form, demographics survey and reply-paid envelope. Written informed consent (return of completed consent form) will be obtained from all participants. No participant will start the trial until consent is received.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous