Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma
- PMID: 30139780
- PMCID: PMC6203407
- DOI: 10.1183/13993003.00936-2018
Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma
Abstract
Benralizumab is an anti-eosinophilic monoclonal antibody that reduces exacerbations and improves lung function for patients with severe, uncontrolled asthma with eosinophilic inflammation. We evaluated the impact of baseline factors on benralizumab efficacy for patients with severe asthma.This analysis used pooled data from the SIROCCO (ClinicalTrials.gov identifier NCT01928771) and CALIMA (ClinicalTrials.gov identifier NCT01914757) Phase III studies. Patients aged 12-75 years with severe, uncontrolled asthma receiving high-dosage inhaled corticosteroids plus long-acting β2-agonists received benralizumab 30 mg subcutaneously every 8 weeks (Q8W, first three doses every 4 weeks (Q4W)), Q4W or placebo. Baseline factors that influenced benralizumab efficacy were evaluated, including oral corticosteroid (OCS) use, nasal polyposis, pre-bronchodilator forced vital capacity (FVC), prior year exacerbations and age at diagnosis. Efficacy outcomes included annual exacerbation rate and change in pre-bronchodilator forced expiratory volume in 1 s at treatment end relative to placebo.Benralizumab Q8W treatment effect was enhanced with each baseline factor for all patients and those with ≥300 eosinophils·μL-1 relative to the overall population. OCS use, nasal polyposis and FVC <65% of predicted were associated with greater benralizumab Q8W responsiveness for reduced exacerbation rate for patients with <300 eosinophils·μL-1Baseline clinical factors and blood eosinophil counts can help identify patients potentially responsive to benralizumab.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: E.R. Bleecker has performed clinical trials through his former employer, the Wake Forest School of Medicine, and his current employer, the University of Arizona; and has served as a paid consultant for AstraZeneca/MedImmune, Boehringer Ingelheim, GSK, Novartis, Regeneron and Sanofi-Genzyme. Conflict of interest: M.E. Wechsler received consulting honoraria from AstraZeneca, Boehringer Ingelheim, Genentech, GSK, Novartis, Regeneron, Sanofi and Teva. Conflict of interest: J.M. FitzGerald is an advisory board member for ALK, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Sanofi-Regeneron and Teva, and has received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Cephalon/Teva, Forest, Genentech, GSK, Johnson and Johnson (Janssen), MedImmune, Novartis, Pfizer and Sanofi. Conflict of interest: A. Menzies-Gow has consultancy agreements with AstraZeneca and Vectura, was an advisory board member for AstraZeneca, Boehringer Ingelheim, GSK, Novartis and Teva, received speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Teva, and Vectura; has participated in research that his institution has been renumerated from Boehringer Ingelheim, GSK and Hoffman La Roche, and has attended international conferences sponsored by AstraZeneca and Boehringer Ingelheim. Conflict of interest: Y. Wu is an employee of AstraZeneca. Conflict of interest: I. Hirsch is an employee of AstraZeneca. Conflict of interest: M. Goldman is an employee of AstraZeneca. Conflict of interest: P. Newbold is an employee of MedImmune LLC. Conflict of interest: J.G. Zangrilli is an employee of AstraZeneca.
Figures

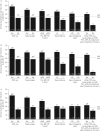

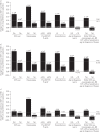


Comment in
-
Exacerbation Rate Reduction With Mepolizumab Stratified by Maintenance Oral Corticosteroids Use and Eosinophil Levels: A Post Hoc Analysis of the DREAM and MENSA Studies.J Investig Allergol Clin Immunol. 2022 Apr 19;32(2):148-150. doi: 10.18176/jiaci.0714. Epub 2021 Jun 4. J Investig Allergol Clin Immunol. 2022. PMID: 34085935 No abstract available.
References
-
- Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy 2012; 42: 650–658. - PubMed
-
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18: 716–725. - PubMed
-
- Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. - PubMed
-
- O'Byrne PM, Pedersen S, Schatz M, et al. . The poorly explored impact of uncontrolled asthma. Chest 2013; 143: 511–523. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
