Single-cell heterogeneity in Sézary syndrome
- PMID: 30139925
- PMCID: PMC6113609
- DOI: 10.1182/bloodadvances.2018022608
Single-cell heterogeneity in Sézary syndrome
Abstract
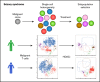
Sézary syndrome (SS) is an aggressive leukemic variant of cutaneous T-cell lymphoma (CTCL) with a median life expectancy of less than 4 years. Although initial treatment responses are often good, the vast majority of patients with SS fail to respond to ongoing therapy. We hypothesize that malignant T cells are highly heterogeneous and harbor subpopulations of SS cells that are both sensitive and resistant to treatment. Here, we investigate the presence of single-cell heterogeneity and resistance to histone deacetylase inhibitors (HDACi) within primary malignant T cells from patients with SS. Using single-cell RNA sequencing and flow cytometry, we find that malignant T cells from all investigated patients with SS display a high degree of single-cell heterogeneity at both the mRNA and protein levels. We show that this heterogeneity divides the malignant cells into distinct subpopulations that can be isolated by their expression of different surface antigens. Finally, we show that treatment with HDACi (suberanilohydroxamic acid and romidepsin) selectively eliminates some subpopulations while leaving other subpopulations largely unaffected. In conclusion, we show that patients with SS display a high degree of single-cell heterogeneity within the malignant T-cell population, and that distinct subpopulations of malignant T cells carry HDACi resistance. Our data point to the importance of understanding the heterogeneous nature of malignant SS cells in each individual patient to design combinational and new therapies to counter drug resistance and treatment failure.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Agar NS, Wedgeworth E, Crichton S, et al. . Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730-4739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

