Impact of Side Chain Polarity on Non-Stoichiometric Nano-Hydroxyapatite Surface Functionalization with Amino Acids
- PMID: 30140033
- PMCID: PMC6107576
- DOI: 10.1038/s41598-018-31058-5
Impact of Side Chain Polarity on Non-Stoichiometric Nano-Hydroxyapatite Surface Functionalization with Amino Acids
Abstract
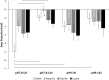
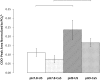
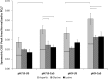
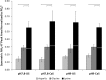
In this study the affinity of three amino acids for the surface of non-stoichiometric hydroxyapatite nanoparticles (ns-nHA) was investigated under different reaction conditions. The amino acids investigated were chosen based on their differences in side chain polarity and potential impact on this surface affinity. While calcium pre-saturation of the calcium-deficient ns-nHA was not found to improve attachment of any of the amino acids studied, the polarity and fraction of ionized functional side groups was found to have a significant impact on this attachment. Overall, amino acid attachment to ns-nHA was not solely reliant on carboxyl groups. In fact, it seems that amine groups also notably interacted with the negative ns-nHA surface and increased the degree of surface binding achieved. As a result, glycine and lysine had greater attachment to ns-nHA than aspartic acid under the reaction conditions studied. Lastly, our results suggest that a layer of each amino acid forms at the surface of ns-nHA, with aspartic acid attachment the most stable and its surface coverage the least of the three amino acids studied.
Conflict of interest statement
The authors declare no competing interests.
Figures
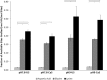
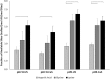


References
-
- Gonzalez-McQuire R, Chane-Ching JY, Vignaud E, Lebugle A, Mann S. Synthesis and characterization of amino acid-functionalized hydroxyapatite nanorods. J. Mater. Chem. 2004;14:2277–2281. doi: 10.1039/b400317a. - DOI
-
- Jack, K. S., Vizcarra, T. G. & Trau, M. Characterization and Surface Properties of Amino-Acid-Modified Carbonate-Containing Hydroxyapatite Particles. Langmuir 12233–12242, 10.1021/la701848c (2007). - PubMed
-
- Ivanova TI, Frank-Kamenetskaya OV, Kol’tsov AB, Ugolkov VL. Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal decomposition. J. Solid State Chem. 2001;160:340–349. doi: 10.1006/jssc.2000.9238. - DOI
-
- Koutsopoulos S, Dalas E. Hydroxyapatite crystallization in the presence of amino acids with uncharged polar side groups: glycine, cysteine, cystine, and glutamine. Langmuir. 2001;17:1074–1079. doi: 10.1021/la000820p. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

