Favorable Response to Treatment with Avelumab in an HIV-Positive Patient with Advanced Merkel Cell Carcinoma Previously Refractory to Chemotherapy
- PMID: 30140209
- PMCID: PMC6103370
- DOI: 10.1159/000490636
Favorable Response to Treatment with Avelumab in an HIV-Positive Patient with Advanced Merkel Cell Carcinoma Previously Refractory to Chemotherapy
Abstract
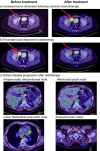
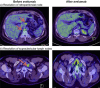
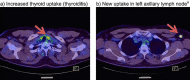
Avelumab is indicated for the management of Merkel cell carcinoma, a rare and aggressive neuroendocrine skin cancer. Its regulatory approval followed the positive outcome of a Phase 2 trial on 88 patients with stage IV disease, which excluded patients with immunodeficiency due to HIV, a risk factor for this cancer type. We report a positive and sustained response to avelumab in an HIV-positive patient with stage IV Merkel cell carcinoma refractory to previous chemotherapy (cisplatin/etoposide) and radiotherapy. Five cycles of avelumab 10 mg/m2 resulted in the resolution of tumor activity visualized using PET-CT scanning in all affected lymph nodes. The only major side effect associated with avelumab was thyroiditis and mild hypothyroidism, a known adverse effect of this treatment, which was well controlled by L-thyroxine treatment. Treatment is ongoing and the positive response has been sustained during 5 further cycles of treatment up to date. This apparently durable response is consistent with the earlier clinical trial experience with avelumab, but seen here in a patient with HIV-associated immunodeficiency as a predisposing factor (an exclusion criterion from the previous trial). Further clinical trials with avelumab in a broader patient population with Merkel cell carcinoma are warranted.
Keywords: Avelumab; Immunotherapy; Merkel cell carcinoma.
Figures
References
-
- Schadendorf D, Lebbé C, Zur Hausen A, Avril MF, Hariharan S, Bharmal M, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017 Jan;71:53–69. - PubMed
-
- American Cancer Society Key statistics for Merkel cell carcinoma Available at https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statisti... (accessed May 2018).
-
- American Cancer Society Survival Rates for Merkel Cell Carcinoma, by Stage Available at https://www.cancer.org/cancer/merkel-cell-skin-cancer/detection-diagnosi... (accessed May 2018).
-
- American Cancer Society Survival Rates for Melanoma Skin Cancer, by Stage. Available at https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-s... (accessed May 2018).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

