Myeloid-Specific Deletion of Peptidylarginine Deiminase 4 Mitigates Atherosclerosis
- PMID: 30140264
- PMCID: PMC6094966
- DOI: 10.3389/fimmu.2018.01680
Myeloid-Specific Deletion of Peptidylarginine Deiminase 4 Mitigates Atherosclerosis
Abstract
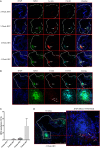
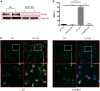
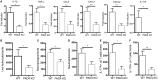
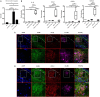
Increasing evidence suggests that neutrophil extracellular traps (NETs) may play a role in promoting atherosclerotic plaque lesions in humans and in murine models. The exact pathways involved in NET-driven atherogenesis remain to be systematically characterized. To assess the extent to which myeloid-specific peptidylarginine deiminase 4 (PAD4) and PAD4-dependent NET formation contribute to atherosclerosis, mice with myeloid-specific deletion of PAD4 were generated and backcrossed to Apoe-/- mice. The kinetics of atherosclerosis development were determined. NETs, but not macrophage extracellular traps, were present in atherosclerotic lesions as early as 3 weeks after initiating high-fat chow. The presence of NETs was associated with the development of atherosclerosis and with inflammatory responses in the aorta. Specific deletion of PAD4 in the myeloid lineage significantly reduced atherosclerosis burden in association with diminished NET formation and reduced inflammatory responses in the aorta. NETs stimulated macrophages to synthesize inflammatory mediators, including IL-1β, CCL2, CXCL1, and CXCL2. Our data support the notion that NETs promote atherosclerosis and that the use of specific PAD4 inhibitors may have therapeutic benefits in this potentially devastating condition.
Keywords: atherosclerosis; inflammation; macrophages; neutrophil extracellular traps; peptidylarginine deiminase 4.
Figures



References
-
- Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res (2015) 116(7):1182–92.10.1161/CIRCRESAHA.116.304944 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

