Survival, Retention, and Selective Proliferation of Lymphocytes Is Mediated by Gingival Fibroblasts
- PMID: 30140265
- PMCID: PMC6094995
- DOI: 10.3389/fimmu.2018.01725
Survival, Retention, and Selective Proliferation of Lymphocytes Is Mediated by Gingival Fibroblasts
Abstract
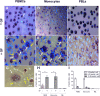
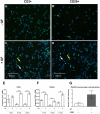
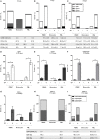
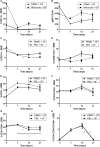
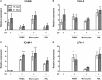
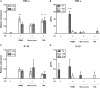
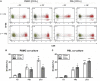
Periodontitis, a chronic inflammatory disease of the periodontium, is characterized by osteoclast-mediated alveolar bone destruction. Gingival fibroblasts (GFs) present in the bone-lining mucosa have the capacity to activate the formation of osteoclasts, but little is known about which local immune cells (co-)mediate this process. The aim of this study was to investigate the cellular interactions of GFs with immune cells, including the contribution of GFs to osteoclast formation and their possible role in the proliferation of these immune cells. In addition, we investigated the expression of adhesion molecules and the inflammatory cytokines that are evoked by this interaction. GFs were cocultured with peripheral blood mononuclear cells (PBMCs), CD14+ monocytes or peripheral blood lymphocytes (PBLs) for 7, 14, and 21 days. After 21 days, comparable numbers of multinucleated cells (osteoclasts) were found in gingival fibroblast (GF)-PBMC and GF-monocyte cocultures. No osteoclasts were formed in GF-PBL cocultures, indicating that the PBLs present in GF-PBMC cocultures do not contribute to osteoclastogenesis. Persisting mononuclear cells were interacting with osteoclasts in GF-PBMC cocultures. Remarkably, a predominance of CD3+ T cells was immunohistochemically detected in GF cocultures with PBLs and PBMCs for 21 days that frequently interacted with osteoclasts. Significantly more T, B (CD19+), and NK (CD56+CD3-) cells were identified with multicolor flow cytometry in both GF-PBMC and GF-PBL cocultures compared to monocultures without GFs at all time points. GFs retained PBLs independently of the presence of monocytes or osteoclasts over time, showing a stable population of T, B, and NK cells between 7 and 21 days. T helper and cytotoxic T cell subsets remained stable over time in GF cocultures, while the number of Th17 cells fluctuated. Lymphocyte retention is likely mediated by lymphocyte-function-associated antigen-1 (LFA-1) expression, which was significantly higher in GF-PBL cultures compared to GF-monocyte cultures. When assessing inflammatory cytokine expression, high tumor necrosis alpha expression was only observed in the GF-PBMC cultures, indicating that this tripartite presence of GFs, monocytes, and lymphocytes is required for such an induction. Carboxyfluorescein succinimidyl ester-labeling showed that only the CD3+ cells proliferated in presence of GFs. This study demonstrates a novel role for GFs in the survival, retention, and selective proliferation of lymphocytes.
Keywords: cell–cell interaction; gingival fibroblast; leukocytes; osteoclast formation; osteoimmunology; peripheral blood cells.
Figures

Similar articles
-
Osteogenic Differentiation of Human Gingival Fibroblasts Inhibits Osteoclast Formation.Cells. 2024 Jun 24;13(13):1090. doi: 10.3390/cells13131090. Cells. 2024. PMID: 38994943 Free PMC article.
-
T Cell Proliferation Is Induced by Chronically TLR2-Stimulated Gingival Fibroblasts or Monocytes.Int J Mol Sci. 2019 Dec 5;20(24):6134. doi: 10.3390/ijms20246134. Int J Mol Sci. 2019. PMID: 31817424 Free PMC article.
-
Gingival fibroblasts are better at inhibiting osteoclast formation than periodontal ligament fibroblasts.J Cell Biochem. 2006 May 15;98(2):370-82. doi: 10.1002/jcb.20795. J Cell Biochem. 2006. PMID: 16440316
-
The dual-functional capability of cytokine-induced killer cells and application in tumor immunology.Hum Immunol. 2015 May;76(5):385-91. doi: 10.1016/j.humimm.2014.09.021. Epub 2014 Oct 8. Hum Immunol. 2015. PMID: 25305457 Review.
-
The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis.J Dent Res. 2023 May;102(5):489-496. doi: 10.1177/00220345231151921. Epub 2023 Mar 8. J Dent Res. 2023. PMID: 36883660 Free PMC article. Review.
Cited by
-
Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement.Int J Mol Sci. 2019 Apr 29;20(9):2108. doi: 10.3390/ijms20092108. Int J Mol Sci. 2019. PMID: 31035654 Free PMC article.
-
The Osteocyte as a Novel Key Player in Understanding Periodontitis Through its Expression of RANKL and Sclerostin: a Review.Curr Osteoporos Rep. 2019 Jun;17(3):116-121. doi: 10.1007/s11914-019-00509-x. Curr Osteoporos Rep. 2019. PMID: 30924022 Free PMC article. Review.
-
Osteogenic Differentiation of Human Gingival Fibroblasts Inhibits Osteoclast Formation.Cells. 2024 Jun 24;13(13):1090. doi: 10.3390/cells13131090. Cells. 2024. PMID: 38994943 Free PMC article.
-
The Challenge of Teaching Essential Immunology Laboratory Skills to Undergraduates in One Month-Experience of an Osteoimmunology Course on TLR Activation.Front Immunol. 2019 Jul 31;10:1822. doi: 10.3389/fimmu.2019.01822. eCollection 2019. Front Immunol. 2019. PMID: 31417577 Free PMC article.
-
T Cell Proliferation Is Induced by Chronically TLR2-Stimulated Gingival Fibroblasts or Monocytes.Int J Mol Sci. 2019 Dec 5;20(24):6134. doi: 10.3390/ijms20246134. Int J Mol Sci. 2019. PMID: 31817424 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

