Molecular Analysis of katG Encoding Catalase-Peroxidase from Clinical Isolate of Isoniazid-Resistant Mycobacterium tuberculosis
- PMID: 30140323
- PMCID: PMC6101688
Molecular Analysis of katG Encoding Catalase-Peroxidase from Clinical Isolate of Isoniazid-Resistant Mycobacterium tuberculosis
Abstract
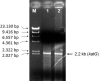
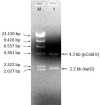
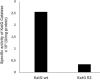
Isoniazid (INH) is a drug for the treatment of tuberculosis in patients infected with Mycobacterium tuberculosis. The katG enzyme, or catalase-peroxidase, activates the pro-drug INH that is coded by the katG gene in M. tuberculosis. Mutations of the katG gene in M. tuberculosis are a major INH resistance mechanism. The M. tuberculosis clinical isolate R2 showed INH resistance at a high level of 10 µg/mL. However, the molecular basis for the resistance is unclear. The identification of a mutation in the katG gene of the clinical isolate R2 showed four mutations, i.e., C1061T, G1261 A, G1388T, G2161A, which correspond to the amino acid substitutions T354I, G421S, R463L, and V721M, respectively. The mutant katG gene, along with the wild-type were cloned, expressed and purified. The mutant enzyme showed 86.5% of catalase and 45% of peroxidase activities in comparison to the wild type. The substitutions of T354I and G421S in mutant katG R2 created significant instability in the adduct triad complex (Trp107-Tyr229-Met255), a part of the active site of the catalase-peroxidase enzyme in the model structure analysis. The events could be based on the high resistance of the clinical isolate R2 toward INH as the molecular basis.
Keywords: Mycobacterium tuberculosis; catalase-peroxidase; isoniazid; katG.
Figures





References
-
- Anonymous. Tuberculosis. http://www.who.int/tb/areas-of-work/drug-resistant-tb/. Retrieved 2018-02-22.
-
- Purkan Ihsanawati, Natalia D, Syah YM, DS Retnoningrum, HS Kusuma. Mutation of katG in a clinical isolate of Mycobacterium tuberculosis: effects on catalase-peroxidase for isoniazid activation. Ukr Biochem J. 2016;88(5):71–81. - PubMed
-
- Heifets LB. Antimycobacterial drugs. Semin. Respir. Infect. 1994;9:84–103. - PubMed
-
- Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. - PubMed
-
- Rozwarski DA, Granst GA, Barton DHR, Jacobs WR, Sacchettini JC. Modification of The NADH of The isoniazid Target (InhA) from Mycobacterium tuberculosis. Science. 1998;279:98–102. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
