Interaction of viral oncogenic proteins with the Wnt signaling pathway
- PMID: 30140402
- PMCID: PMC6098952
- DOI: 10.22038/IJBMS.2018.28903.6982
Interaction of viral oncogenic proteins with the Wnt signaling pathway
Abstract
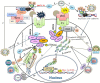
It is estimated that up to 20% of all types of human cancers worldwide are attributed to viruses. The genome of oncogenic viruses carries genes that have protein products that act as oncoproteins in cell proliferation and transformation. The modulation of cell cycle control mechanisms, cellular regulatory and signaling pathways by oncogenic viruses, plays an important role in viral carcinogenesis. Different signaling pathways play a part in the carcinogenesis that occurs in a cell. Among these pathways, the Wnt signaling pathway plays a predominant role in carcinogenesis and is known as a central cellular pathway in the development of tumors. There are three Wnt signaling pathways that are well identified, including the canonical or Wnt/β-catenin dependent pathway, the noncanonical or β-catenin-independent planar cell polarity (PCP) pathway, and the noncanonical Wnt/Ca2+ pathway. Most of the oncogenic viruses modulate the canonical Wnt signaling pathway. This review discusses the interaction between proteins of several human oncogenic viruses with the Wnt signaling pathway.
Keywords: Canonical pathway; Carcinogenesis; Oncogenic viruses; Wnt signaling; β-catenin.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Comparative Risk Assessment collaborating g Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. - PubMed
-
- Niller HH, Wolf H, Minarovits J. Viral hit and run-oncogenesis: genetic and epigenetic scenarios. Cancer Lett. 2011;305:200–217. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
