A high-throughput microtissue platform to probe endothelial function in vitro
- PMID: 30140833
- PMCID: PMC6558661
- DOI: 10.1039/c8ib00111a
A high-throughput microtissue platform to probe endothelial function in vitro
Abstract
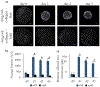
A critical role of vascular endothelium is as a semi-permeable barrier, dynamically regulating the flux of solutes between blood and the surrounding tissue. Existing platforms that quantify endothelial function in vitro are either significantly throughput limited or overlook physiologically relevant extracellular matrix (ECM) interactions and thus do not recapitulate in vivo function. Leveraging droplet microfluidics, we developed a scalable platform to measure endothelial function in nanoliter-volume, ECM-based microtissues. In this study, we describe our high-throughput method for fabricating endothelial-coated collagen microtissues that incorporate physiologically relevant cell-ECM interactions. We showed that the endothelial cells had characteristic morphology, expressed tight junction proteins, and remodeled the ECM via compaction and deposition of basement membrane. We also measured macromolecular permeability using two optical modalities, and found the cell layers: (1) had permeability values comparable to in vivo measurements and (2) were responsive to physiologically-relevant modulators of endothelial permeability (TNF-α and TGF-β). This is the first demonstration, to the authors' knowledge, of high-throughput assessment (n > 150) of endothelial permeability on natural ECM. Additionally, this technology is compatible with standard cell culture equipment (e.g. multi-well plates) and could be scaled up further to be integrated with automated liquid handling systems and automated imaging platforms. Overall, this platform recapitulates the functions of traditional transwell inserts, but extends application to high-throughput studies and introduces new possibilities for interrogating cell-cell and cell-matrix interactions.
Conflict of interest statement
Conflicts of Interest:
There are no conflicts to declare.
Figures




References
-
- Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992. May 1;6(8):2591–9. - PubMed
-
- Friedman M, Byers SO. Endothelial permeability in atherosclerosis. Arch Pathol. 1963;76:99–105. - PubMed
-
- Veress B, Bálint A, Kóczé A, Nagy Z, Jellinek H. Increasing aortic permeability by atherogenic diet. Atherosclerosis. 1970. May 1;11(3):369–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

