Changes in proteome solubility indicate widespread proteostatic disruption in mouse models of neurodegenerative disease
- PMID: 30140941
- PMCID: PMC6411038
- DOI: 10.1007/s00401-018-1895-y
Changes in proteome solubility indicate widespread proteostatic disruption in mouse models of neurodegenerative disease
Abstract
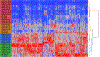
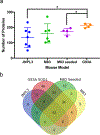
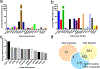
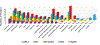
The deposition of pathologic misfolded proteins in neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, frontotemporal dementia and amyotrophic lateral sclerosis is hypothesized to burden protein homeostatic (proteostatic) machinery, potentially leading to insufficient capacity to maintain the proteome. This hypothesis has been supported by previous work in our laboratory, as evidenced by the perturbation of cytosolic protein solubility in response to amyloid plaques in a mouse model of Alzheimer's amyloidosis. In the current study, we demonstrate changes in proteome solubility are a common pathology to mouse models of neurodegenerative disease. Pathological accumulations of misfolded tau, α-synuclein and mutant superoxide dismutase 1 in CNS tissues of transgenic mice were associated with changes in the solubility of hundreds of CNS proteins in each model. We observed that changes in proteome solubility were progressive and, using the rTg4510 model of inducible tau pathology, demonstrated that these changes were dependent upon sustained expression of the primary pathologic protein. In all of the models examined, changes in proteome solubility were robust, easily detected, and provided a sensitive indicator of proteostatic disruption. Interestingly, a subset of the proteins that display a shift towards insolubility were common between these different models, suggesting that a specific subset of the proteome is vulnerable to proteostatic disruption. Overall, our data suggest that neurodegenerative proteinopathies modeled in mice impose a burden on the proteostatic network that diminishes the ability of neural cells to prevent aberrant conformational changes that alter the solubility of hundreds of abundant cellular proteins.
Keywords: Neurodegeneration; Protein misfolding; Proteinopathy; Proteomics; Proteostasis.
Conflict of interest statement
Figures



References
-
- Ayyadevara S, Balasubramaniam M, Parcon PA, Barger SW, Griffin WST, Alla R, Tackett AJ, Mackintosh SG, Petricoin E, Zhou W, Shmookler Reis RJ (2016) Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer’s hippocampus from normal controls. Aging Cell 15:924–939. doi: 10.1111/acel.12501 - DOI - PMC - PubMed
-
- Bai B, Hales CM, Chen P, Gozal Y, Dammer EB, Fritz JJ (2013) U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer ‘ s disease. Proc Natl Acad Sci U S A 110:16562–16567. doi: 10.1073/pnas.1310249110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1310249110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

