Effects of program scale-up on time to resolution for patients with abnormal screening mammography results
- PMID: 30140972
- PMCID: PMC6162097
- DOI: 10.1007/s10552-018-1074-4
Effects of program scale-up on time to resolution for patients with abnormal screening mammography results
Abstract
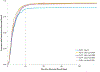
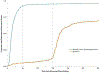
Purpose: Effects of geographic program expansion to rural areas on screening program outcomes are understudied. We sought to determine whether time-to-resolution (TTR) varied significantly by service delivery time period, location, and participant characteristics across 19 North Texas counties.
Methods: We calculated proportions undergoing diagnostic follow-up and resolved ≤ 60 days. We calculated median TTR for each time period and abnormal result BI-RADS 0, 4, 5. Cox proportional hazards regressions estimated time period and patient characteristic effects on TTR. Wilcoxon rank sum tests evaluated whether TTR differed between women who did or did not transfer between counties for services.
Results: TTR ranged from 14 to 17 days for BI-RADs 0, 4, and 5; 12.4% transferred to a different county, resulting in longer median TTR (26 vs. 16 days; p < .001). Of those completing follow-up, 92% were resolved ≤ 60 days (median 15 days). For BI-RAD 3, TTR was 208 days (including required 180 day waiting period). Follow-up was significantly lower for women with BI-RAD 3 (59% vs. 96%; p < .0001).
Conclusion: Expansion maintained timely service delivery, increasing access to screening among rural, uninsured women. Policies adding a separate quality metric for BI-RAD 3 could encourage follow-up monitoring to address lower completion and longer TTR among women with this result.
Keywords: Breast cancer screening; Quality; Rural; Scale-up.
Conflict of interest statement
All authors declare no conflicts of interest
Figures

Similar articles
-
Time to diagnostic resolution after an uncertain screening mammogram in an underserved population.Cancer Med. 2020 May;9(9):3252-3258. doi: 10.1002/cam4.2970. Epub 2020 Mar 11. Cancer Med. 2020. PMID: 32160406 Free PMC article.
-
Patient navigation to improve follow-up of abnormal mammograms among disadvantaged women.J Womens Health (Larchmt). 2015 Feb;24(2):138-43. doi: 10.1089/jwh.2014.4954. Epub 2014 Dec 18. J Womens Health (Larchmt). 2015. PMID: 25522246 Free PMC article.
-
[Tailored Breast Screening Trial (TBST)].Epidemiol Prev. 2013 Jul-Oct;37(4-5):317-27. Epidemiol Prev. 2013. PMID: 24293498 Clinical Trial. Italian.
-
[Analysis of the results of mammography screening in Dubrovnik-Neretva County in the 2006-2009 period].Acta Med Croatica. 2010 Dec;64(5):453-9. Acta Med Croatica. 2010. PMID: 21692270 Croatian.
-
Evaluation of abnormal mammography results and palpable breast abnormalities.Ann Intern Med. 2003 Aug 19;139(4):274-84. doi: 10.7326/0003-4819-139-4-200308190-00010. Ann Intern Med. 2003. PMID: 12965983 Review.
Cited by
-
Facilitators of Multisector Collaboration for Delivering Cancer Control Interventions in Rural Communities: A Descriptive Qualitative Study.Prev Chronic Dis. 2022 Aug 11;19:E48. doi: 10.5888/pcd19.210450. Prev Chronic Dis. 2022. PMID: 35951440 Free PMC article.
-
Time to diagnostic resolution after an uncertain screening mammogram in an underserved population.Cancer Med. 2020 May;9(9):3252-3258. doi: 10.1002/cam4.2970. Epub 2020 Mar 11. Cancer Med. 2020. PMID: 32160406 Free PMC article.
-
Long-term Mammography Adherence among Uninsured Women Enrolled in the Breast Screening and Patient Navigation (BSPAN) Program.Cancer Epidemiol Biomarkers Prev. 2022 Jan;31(1):77-84. doi: 10.1158/1055-9965.EPI-21-0191. Epub 2021 Nov 8. Cancer Epidemiol Biomarkers Prev. 2022. PMID: 34750203 Free PMC article.
References
-
- Elmore JG. (2016) Review: Mammography screening reduces breast cancer mortality in women at average risk. Ann Intern Med. 164: Jc26. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

