Immune Response to Human Papillomavirus One Year after Prophylactic Vaccination with AS04-Adjuvanted HPV-16/18 Vaccine: HPV-Specific IgG and IgA Antibodies in the Circulation and the Cervix
- PMID: 30141308
- PMCID: PMC6171383
- DOI: 10.22034/APJCP.2018.19.8.2313
Immune Response to Human Papillomavirus One Year after Prophylactic Vaccination with AS04-Adjuvanted HPV-16/18 Vaccine: HPV-Specific IgG and IgA Antibodies in the Circulation and the Cervix
Abstract
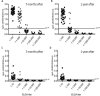
Objective: This study was designed to describe the course of IgG/IgA responses in cervical secretions and in serum one year after the first dose of intramuscular administration of the HPV16/18 AS04-adjuvant vaccine. Methods: Blood and cervical mucus samples were collected for immunologic assays, 7 months after the first doses and 1 year following the last boost vaccination (month 7) by enzyme linked immunosorbent assay (ELISA). The detection of IgG and IgA anti-HPV/VLP was developed for this purpose. Result: A total of 100% of serum samples were IgG antibody positive at a titer of 1:100 at both time periods and decreased according to the serum dilution. For serum IgA antibody, 95% were positive one month after vaccination and 79% were positive 1 year later. Similar results were observed with the cervical samples positive for both IgG and IgA antibodies at one month and decreasing after 1 year to 33% and 29%. The median absorbance in serum and the cervix for IgG and IgA anti-HPV-VLP antibodies was significantly higher at one month after vaccination when compared to 1 year post-vaccination (P<0.0001). Conclusion: Immune responses were significant one year after immunization, however it decreased in cervical and serum samples when compared to levels observed one month after the last dose. This suggests that a vaccine booster may be necessary to increase antibody titers.
Keywords: Immunoglobulin G; immunoglobulin A; HPV; vaccine.
Creative Commons Attribution License
Figures

Similar articles
-
Infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions. A review of the literature.Papillomavirus Res. 2019 Dec;8:100185. doi: 10.1016/j.pvr.2019.100185. Epub 2019 Sep 5. Papillomavirus Res. 2019. PMID: 31494291 Free PMC article. Review.
-
Characterization of Immunoglobulin A/G Responses During 3 Doses of the Human Papillomavirus-16/18 ASO4-Adjuvanted Vaccine.Sex Transm Dis. 2016 May;43(5):335-9. doi: 10.1097/OLQ.0000000000000429. Sex Transm Dis. 2016. PMID: 27100772
-
Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women.Int J Cancer. 2011 Nov 1;129(9):2147-57. doi: 10.1002/ijc.25887. Epub 2011 Mar 11. Int J Cancer. 2011. PMID: 21190190 Clinical Trial.
-
Correlation between levels of human papillomavirus (HPV)-16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV-16/18 AS04-adjuvanted vaccine.Hum Vaccin. 2010 Dec;6(12):1054-61. doi: 10.4161/hv.6.12.13399. Epub 2010 Dec 1. Hum Vaccin. 2010. PMID: 21157180 Clinical Trial.
-
AS04-adjuvanted human papillomavirus-16/18 vaccination: recent advances in cervical cancer prevention.Expert Rev Vaccines. 2008 Dec;7(10):1465-73. doi: 10.1586/14760584.7.10.1465. Expert Rev Vaccines. 2008. PMID: 19053203 Review.
Cited by
-
Long-Term Antibody Response and Vaccination Efficacy in Patients with COVID-19: A Single Center One-Year Prospective Study from the Czech Republic.Viruses. 2022 Mar 4;14(3):526. doi: 10.3390/v14030526. Viruses. 2022. PMID: 35336932 Free PMC article.
-
Tityus serrulatus Scorpion Venom Induces Apoptosis in Cervical Cancer Cell Lines.Evid Based Complement Alternat Med. 2019 Jun 23;2019:5131042. doi: 10.1155/2019/5131042. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31341494 Free PMC article.
-
Infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions. A review of the literature.Papillomavirus Res. 2019 Dec;8:100185. doi: 10.1016/j.pvr.2019.100185. Epub 2019 Sep 5. Papillomavirus Res. 2019. PMID: 31494291 Free PMC article. Review.
-
An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy.Front Immunol. 2022 Jan 27;12:805695. doi: 10.3389/fimmu.2021.805695. eCollection 2021. Front Immunol. 2022. PMID: 35154080 Free PMC article. Review.
-
The Tumor-Specific Immune Landscape in HPV+ Head and Neck Cancer.Viruses. 2023 May 31;15(6):1296. doi: 10.3390/v15061296. Viruses. 2023. PMID: 37376596 Free PMC article. Review.
References
-
- Dempsey AF, Brewer SE, Pyrzanowski J, et al. Acceptability of human papillomavirus vaccines among women older than 26 years. Vaccine. 2015;33:1556–61. - PubMed
-
- Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women:a randomized clinical trial. JAMA. 2013;309:1793–802. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

