Chemoenzymatic Methods for the Synthesis of Glycoproteins
- PMID: 30141327
- PMCID: PMC6238085
- DOI: 10.1021/acs.chemrev.8b00238
Chemoenzymatic Methods for the Synthesis of Glycoproteins
Abstract
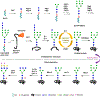
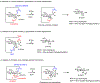
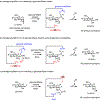
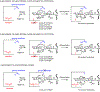
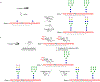
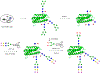
Glycosylation is one of the most prevalent posttranslational modifications that profoundly affects the structure and functions of proteins in a wide variety of biological recognition events. However, the structural complexity and heterogeneity of glycoproteins, usually resulting from the variations of glycan components and/or the sites of glycosylation, often complicates detailed structure-function relationship studies and hampers the therapeutic applications of glycoproteins. To address these challenges, various chemical and biological strategies have been developed for producing glycan-defined homogeneous glycoproteins. This review highlights recent advances in the development of chemoenzymatic methods for synthesizing homogeneous glycoproteins, including the generation of various glycosynthases for synthetic purposes, endoglycosidase-catalyzed glycoprotein synthesis and glycan remodeling, and direct enzymatic glycosylation of polypeptides and proteins. The scope, limitation, and future directions of each method are discussed.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures





















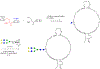






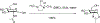









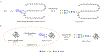
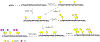
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

