Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca2+ signaling in vascular endothelial cells
- PMID: 30141983
- PMCID: PMC6297820
- DOI: 10.1152/ajpheart.00204.2018
Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca2+ signaling in vascular endothelial cells
Abstract
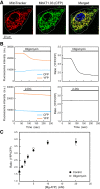
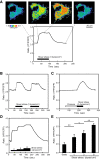
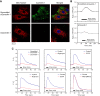
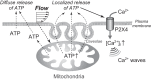
Vascular endothelial cells (ECs) sense and transduce hemodynamic shear stress into intracellular biochemical signals, and Ca2+ signaling plays a critical role in this mechanotransduction, i.e., ECs release ATP in the caveolae in response to shear stress and, in turn, the released ATP activates P2 purinoceptors, which results in an influx into the cells of extracellular Ca2+. However, the mechanism by which the shear stress evokes ATP release remains unclear. Here, we demonstrated that cellular mitochondria play a critical role in this process. Cultured human pulmonary artery ECs were exposed to controlled levels of shear stress in a flow-loading device, and changes in the mitochondrial ATP levels were examined by real-time imaging using a fluorescence resonance energy transfer-based ATP biosensor. Immediately upon exposure of the cells to flow, mitochondrial ATP levels increased, which was both reversible and dependent on the intensity of shear stress. Inhibitors of the mitochondrial electron transport chain and ATP synthase as well as knockdown of caveolin-1, a major structural protein of the caveolae, abolished the shear stress-induced mitochondrial ATP generation, resulting in the loss of ATP release and influx of Ca2+ into the cells. These results suggest the novel role of mitochondria in transducing shear stress into ATP generation: ATP generation leads to ATP release in the caveolae, triggering purinergic Ca2+ signaling. Thus, exposure of ECs to shear stress seems to activate mitochondrial ATP generation through caveola- or caveolin-1-mediated mechanisms. NEW & NOTEWORTHY The mechanism of how vascular endothelial cells sense shear stress generated by blood flow and transduce it into functional responses remains unclear. Real-time imaging of mitochondrial ATP demonstrated the novel role of endothelial mitochondria as mechanosignaling organelles that are able to transduce shear stress into ATP generation, triggering ATP release and purinoceptor-mediated Ca2+ signaling within the cells.
Keywords: ATP; calcium signaling; endothelial cells; mitochondria; shear stress.
Figures

Similar articles
-
Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells.J Cell Sci. 2011 Oct 15;124(Pt 20):3477-83. doi: 10.1242/jcs.087221. J Cell Sci. 2011. PMID: 22010198
-
Shear stress activates mitochondrial oxidative phosphorylation by reducing plasma membrane cholesterol in vascular endothelial cells.Proc Natl Acad Sci U S A. 2020 Dec 29;117(52):33660-33667. doi: 10.1073/pnas.2014029117. Epub 2020 Dec 14. Proc Natl Acad Sci U S A. 2020. PMID: 33318210 Free PMC article.
-
Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells.Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1646-53. doi: 10.1152/ajpheart.01385.2006. Epub 2007 Jun 1. Am J Physiol Heart Circ Physiol. 2007. PMID: 17545472
-
[Blood flow sensing mechanism via calcium signaling in vascular endothelium].Yakugaku Zasshi. 2010 Nov;130(11):1407-11. doi: 10.1248/yakushi.130.1407. Yakugaku Zasshi. 2010. PMID: 21048396 Review. Japanese.
-
[Shear-stress sensing via P2 purinoceptors in vascular endothelial cells].Nihon Yakurigaku Zasshi. 2004 Nov;124(5):319-28. doi: 10.1254/fpj.124.319. Nihon Yakurigaku Zasshi. 2004. PMID: 15502397 Review. Japanese.
Cited by
-
Flow-Induced Secretion of Endothelial Heparanase Regulates Cardiac Lipoprotein Lipase and Changes Following Diabetes.J Am Heart Assoc. 2022 Dec 6;11(23):e027958. doi: 10.1161/JAHA.122.027958. Epub 2022 Nov 23. J Am Heart Assoc. 2022. PMID: 36416172 Free PMC article.
-
Microfluidic investigation for shear-stress-mediated repair of dysglycemia-induced endothelial cell damage.Mechanobiol Med. 2024 Apr 29;2(3):100069. doi: 10.1016/j.mbm.2024.100069. eCollection 2024 Sep. Mechanobiol Med. 2024. PMID: 40395495 Free PMC article. Review.
-
Biomechanical and Mechanobiological Drivers of the Transition From PostCapillary Pulmonary Hypertension to Combined Pre-/PostCapillary Pulmonary Hypertension.J Am Heart Assoc. 2023 Feb 7;12(3):e028121. doi: 10.1161/JAHA.122.028121. Epub 2023 Feb 3. J Am Heart Assoc. 2023. PMID: 36734341 Free PMC article. Review.
-
Mechanical forces and metabolic changes cooperate to drive cellular memory and endothelial phenotypes.Curr Top Membr. 2021;87:199-253. doi: 10.1016/bs.ctm.2021.07.003. Epub 2021 Sep 25. Curr Top Membr. 2021. PMID: 34696886 Free PMC article. Review.
-
NTPDase1/CD39 Ectonucleotidase Is Necessary for Normal Arterial Diameter Adaptation to Flow.Int J Mol Sci. 2023 Oct 10;24(20):15038. doi: 10.3390/ijms242015038. Int J Mol Sci. 2023. PMID: 37894719 Free PMC article.
References
-
- Aoki T, Nishimura M, Matsuoka T, Yamamoto K, Furuyashiki T, Kataoka H, Kitaoka S, Ishibashi R, Ishibazawa A, Miyamoto S, Morishita R, Ando J, Hashimoto N, Nozaki K, Narumiya S. PGE2-EP2 signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br J Pharmacol 163: 1237–1249, 2011. doi:10.1111/j.1476-5381.2011.01358.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

