Which neoadjuvant chemotherapy regimen should be recommended for patients with advanced nasopharyngeal carcinoma?: A network meta-analysis
- PMID: 30142830
- PMCID: PMC6112937
- DOI: 10.1097/MD.0000000000011978
Which neoadjuvant chemotherapy regimen should be recommended for patients with advanced nasopharyngeal carcinoma?: A network meta-analysis
Abstract
Background: The clinical application has widespread disagreement on the different regimens of neoadjuvant chemotherapy (NCT) in the treatment of locoregionally advanced nasopharyngeal carcinoma (NPC). We conducted a network meta-analysis (NMA) to evaluate the efficacy of the different NCT regimens in the treatment of NPC.
Methods: A systematic literature search was performed using PubMed, Embase, and Cochran Library. Totally, 31 randomized controlled trials (RCTs) (n = 4062) met study selection criteria and were incorporated in this NMA study.
Results: Our study showed that certain NCT regimens improved the prognosis of patients, and found out the relative best solution for each endpoint, such as paclitaxel, carboplatin, and gemcitabine for 1-year overall survival (OS) rate, cisplatin, calcium folinate, and 5-fluorouracil for 2-year OS rate, vinorelbine and cisplatin (NP) for 3-year OS rate, cyclophosphamide, cisplatin, and 5-fluorouracil for 5-year OS rate, NP for complete remission rate, cisplatin and gemcitabine for overall remission rate of the primary tumor. In addition, for certain grade 3 and above toxicity, the results of the NMA reflected certain NCT regimens can reduce toxicity of chemoradiotherapy (CRT) to a minimum, such as NP for anemia, mucositis, and thrombocytopenia, paclitaxel, epirubicin, and cisplatin for neutropenia and skin toxicity.
Conclusion: Our NMA showed that certain cisplatin-based NCT regimens improved the prognosis of patients with NPC and reduced the toxicity of CRT. However, in view of survival rate and response rate, the best NCT regimen is not entirely consistent. Therefore, which NCT regimen will benefit most patients will need further explored.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


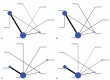

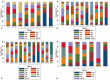
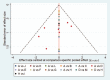
References
-
- Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1765–77. - PubMed
-
- Li JG, Yuan X, Zhang LL, et al. A randomized clinical trial comparing prophylactic upper versus whole-neck irradiation in the treatment of patients with node-negative nasopharyngeal carcinoma. Cancer 2013;119:3170–6. - PubMed
-
- Wang WY, Twu CW, Chen HH, et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer 2013;119:963–70. - PubMed
-
- Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol 2012;22:233–44. - PubMed
-
- OuYang PY, Xie C, Mao YP, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol 2013;24:2136–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

