Radiation Sensitization of Basal Cell and Head and Neck Squamous Cell Carcinoma by the Hedgehog Pathway Inhibitor Vismodegib
- PMID: 30142876
- PMCID: PMC6164565
- DOI: 10.3390/ijms19092485
Radiation Sensitization of Basal Cell and Head and Neck Squamous Cell Carcinoma by the Hedgehog Pathway Inhibitor Vismodegib
Abstract
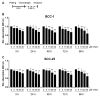
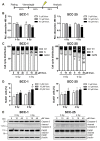
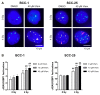
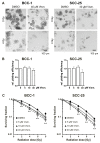
Vismodegib, an inhibitor of the Hedgehog signaling pathway, is an approved drug for monotherapy in locally advanced or metastatic basal cell carcinoma (BCC). Data on combined modality treatment by vismodegib and radiation therapy, however, are rare. In the present study, we examined the radiation sensitizing effects of vismodegib by analyzing viability, cell cycle distribution, cell death, DNA damage repair and clonogenic survival in three-dimensional cultures of a BCC and a head and neck squamous cell carcinoma (HNSCC) cell line. We found that vismodegib decreases expression of the Hedgehog target genes glioma-associated oncogene homologue (GLI1) and the inhibitor of apoptosis protein (IAP) Survivin in a cell line- and irradiation-dependent manner, most pronounced in squamous cell carcinoma (SCC) cells. Furthermore, vismodegib significantly reduced proliferation in both cell lines, while additional irradiation only slightly further impacted on viability. Analyses of cell cycle distribution and cell death induction indicated a G1 arrest in BCC and a G2 arrest in HNSCC cells and an increased fraction of cells in SubG1 phase following combined treatment. Moreover, a significant rise in the number of phosphorylated histone-2AX/p53-binding protein 1 (γH2AX/53BP1) foci in vismodegib- and radiation-treated cells was associated with a significant radiosensitization of both cell lines. In summary, these findings indicate that inhibition of the Hedgehog signaling pathway may increase cellular radiation response in BCC and HNSCC cells.
Keywords: basal cell carcinoma; head and neck squamous cell carcinoma; hedgehog signaling pathway; radiotherapy resistance; vismodegib (GDC-0449).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

