Roles of the TGF-β⁻VEGF-C Pathway in Fibrosis-Related Lymphangiogenesis
- PMID: 30142879
- PMCID: PMC6163754
- DOI: 10.3390/ijms19092487
Roles of the TGF-β⁻VEGF-C Pathway in Fibrosis-Related Lymphangiogenesis
Abstract
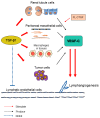
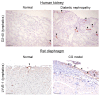
Lymphatic vessels drain excess tissue fluids to maintain the interstitial environment. Lymphatic capillaries develop during the progression of tissue fibrosis in various clinical and pathological situations, such as chronic kidney disease, peritoneal injury during peritoneal dialysis, tissue inflammation, and tumor progression. The role of fibrosis-related lymphangiogenesis appears to vary based on organ specificity and etiology. Signaling via vascular endothelial growth factor (VEGF)-C, VEGF-D, and VEGF receptor (VEGFR)-3 is a central molecular mechanism for lymphangiogenesis. Transforming growth factor-β (TGF-β) is a key player in tissue fibrosis. TGF-β induces peritoneal fibrosis in association with peritoneal dialysis, and also induces peritoneal neoangiogenesis through interaction with VEGF-A. On the other hand, TGF-β has a direct inhibitory effect on lymphatic endothelial cell growth. We proposed a possible mechanism of the TGF-β⁻VEGF-C pathway in which TGF-β promotes VEGF-C production in tubular epithelial cells, macrophages, and mesothelial cells, leading to lymphangiogenesis in renal and peritoneal fibrosis. Connective tissue growth factor (CTGF) is also involved in fibrosis-associated renal lymphangiogenesis through interaction with VEGF-C, in part by mediating TGF-β signaling. Further clarification of the mechanism might lead to the development of new therapeutic strategies to treat fibrotic diseases.
Keywords: fibrosis; lymphangiogenesis; transforming growth factor-β; vascular endothelial growth factor-C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Connective tissue growth factor is correlated with peritoneal lymphangiogenesis.Sci Rep. 2019 Aug 21;9(1):12175. doi: 10.1038/s41598-019-48699-9. Sci Rep. 2019. PMID: 31434958 Free PMC article.
-
TGF-β1 promotes lymphangiogenesis during peritoneal fibrosis.J Am Soc Nephrol. 2013 Oct;24(10):1627-42. doi: 10.1681/ASN.2012030226. Epub 2013 Aug 29. J Am Soc Nephrol. 2013. PMID: 23990681 Free PMC article.
-
Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D.J Biol Chem. 2016 Dec 30;291(53):27265-27278. doi: 10.1074/jbc.M116.736801. Epub 2016 Nov 16. J Biol Chem. 2016. PMID: 27852824 Free PMC article.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
Molecular targeting of lymphatics for therapy.Curr Pharm Des. 2004;10(1):65-74. doi: 10.2174/1381612043453513. Curr Pharm Des. 2004. PMID: 14754406 Review.
Cited by
-
Connective tissue growth factor is correlated with peritoneal lymphangiogenesis.Sci Rep. 2019 Aug 21;9(1):12175. doi: 10.1038/s41598-019-48699-9. Sci Rep. 2019. PMID: 31434958 Free PMC article.
-
Transcription factor A, mitochondrial promotes lymph node metastasis and lymphangiogenesis in epithelial ovarian carcinoma.Open Med (Wars). 2025 Feb 7;20(1):20241089. doi: 10.1515/med-2024-1089. eCollection 2025. Open Med (Wars). 2025. PMID: 39927160 Free PMC article.
-
Lymphatic Endothelial-to-Myofibroblast Transition: A Potential New Mechanism Underlying Skin Fibrosis in Systemic Sclerosis.Cells. 2023 Sep 1;12(17):2195. doi: 10.3390/cells12172195. Cells. 2023. PMID: 37681927 Free PMC article.
-
Expression levels of VEGF-C and VEGFR-3 in renal cell carcinoma and their association with lymph node metastasis.Exp Ther Med. 2021 Jun;21(6):554. doi: 10.3892/etm.2021.9986. Epub 2021 Mar 26. Exp Ther Med. 2021. PMID: 33850526 Free PMC article.
-
Combination of Anti-Angiogenics and Immunotherapies in Renal Cell Carcinoma Show Their Limits: Targeting Fibrosis to Break through the Glass Ceiling?Biomedicines. 2024 Feb 7;12(2):385. doi: 10.3390/biomedicines12020385. Biomedicines. 2024. PMID: 38397987 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

