Randomized Trial of a High Protein, Partial Meal Replacement Program with or without Alternate Day Fasting: Similar Effects on Weight Loss, Retention Status, Nutritional, Metabolic, and Behavioral Outcomes
- PMID: 30142886
- PMCID: PMC6165084
- DOI: 10.3390/nu10091145
Randomized Trial of a High Protein, Partial Meal Replacement Program with or without Alternate Day Fasting: Similar Effects on Weight Loss, Retention Status, Nutritional, Metabolic, and Behavioral Outcomes
Abstract
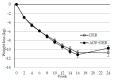
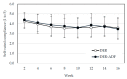
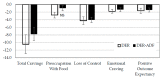
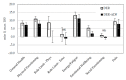
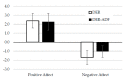
Higher-protein diets, meal replacements, and greater early weight loss have separately been associated with greater weight loss. We compared a high-protein, meal replacement program with daily energy restriction (DER) to one which provided greater energy restriction adding alternate day fasting (ADF + DER; alternating days of modified-fasting and DER plus 1 ad libitum day/week) on retention, weight loss, physiological, nutritional, and behavioral markers. Participants were randomized to ADF + DER or DER for 16 weeks (n = 162, age 40 ± 8 years BMI 36 ± 6 kg/m² (Mean ± SD)) plus 8 weeks weight maintenance. At week 16 weight change was -10.7 ± 0.5 kg and -11.2 ± 0.6 kg in ADF + DER and DER groups (treatment NS). Fat mass, visceral adipose tissue, and lean mass (p < 0.05) were similarly reduced between treatments. Weight loss was sustained to 24 weeks (treatment NS). Fasting LDL-cholesterol, triglycerides, insulin, hsCRP, glucose, and blood pressure all improved (p < 0.05; treatment NS). Transferrin saturation, ferritin, serum zinc, folate, and B12 improved (p < 0.05; treatment NS). Plasma thiamine and vitamin D levels decreased, reflecting lower carbohydrate intakes and seasonal changes, respectively. Food cravings, quality of life, and mood improved (treatment NS). Energy, fatigue, and pain improved slightly more in DER (p < 0.05). This study supports the use of higher protein, meal replacement programs with or without ADF in weight management.
Keywords: fasting; meal replacement; weight loss.
Conflict of interest statement
CSIRO has an ongoing research partnership with Probiotec in order to develop the Impromy™ program. Probiotec had no input into this paper and only offered advice on the trial design regarding the plausibility of the program in a commercial setting. Funding for this trial was provided by CSIRO as well as royalties from the Impromy™ program.
Figures
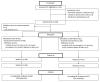
References
-
- Aller E.E.J.G., Larsen T.M., Claus H., Lindroos A.K., Kafatos A., Pfeiffer A., Martinez J.A., Handjieva-Darlenska T., Kunesova M., Stender S., et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: The DIOGENES trial 12 months results. Int. J. Obes. 2014;38:1–33. doi: 10.1038/ijo.2014.52. - DOI - PubMed
-
- Johansson K., Neovius M., Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014;99:14–23. doi: 10.3945/ajcn.113.070052. - DOI - PMC - PubMed
-
- Unick J.L., Neiberg R.H., Hogan P.E., Cheskin L.J., Dutton G.R., Jeffery R., Nelson J.A., Pi-Sunyer X., West D.S., Wing R.R., et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity. 2015;23:1353–1356. doi: 10.1002/oby.21112. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

