Linking Aromatic Hydroxy Metabolic Functionalization of Drug Molecules to Structure and Pharmacologic Activity
- PMID: 30142909
- PMCID: PMC6225321
- DOI: 10.3390/molecules23092119
Linking Aromatic Hydroxy Metabolic Functionalization of Drug Molecules to Structure and Pharmacologic Activity
Abstract
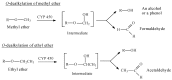
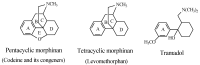
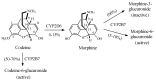
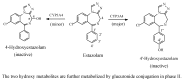
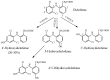
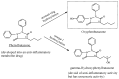
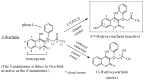
Drug functionalization through the formation of hydrophilic groups is the norm in the phase I metabolism of drugs for the modification of drug action. The reactions involved are mainly oxidative, catalyzed mostly by cytochrome P450 (CYP) isoenzymes. The benzene ring, whether phenyl or fused with other rings, is the most common hydrophobic pharmacophoric moiety in drug molecules. On the other hand, the alkoxy group (mainly methoxy) bonded to the benzene ring assumes an important and sometimes essential pharmacophoric status in some drug classes. Upon metabolic oxidation, both moieties, i.e., the benzene ring and the alkoxy group, produce hydroxy groups; the products are arenolic in nature. Through a pharmacokinetic effect, the hydroxy group enhances the water solubility and elimination of the metabolite with the consequent termination of drug action. However, through hydrogen bonding, the hydroxy group may modify the pharmacodynamics of the interaction of the metabolite with the site of parent drug action (i.e., the receptor). Accordingly, the expected pharmacologic outcome will be enhancement, retention, attenuation, or loss of activity of the metabolite relative to the parent drug. All the above issues are presented and discussed in this review using selected members of different classes of drugs with inferences regarding mechanisms, drug design, and drug development.
Keywords: arenolic drug metabolites; aromatic hydroxy metabolites; auxophores; metabolic O-dealkylation; metabolic aromatic-ring hydroxylation; metabolic modification of drug activity; primary and auxiliary pharmacophores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

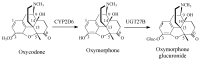





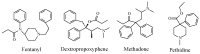
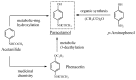
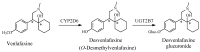


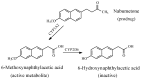







References
-
- The Importance of Stereochemistry in Drug Action and Disposition. [(accessed on 25 July 2018)]; Available online: https://www.researchgate.net/publication/21709119_The_Importance_of_Ster....
-
- Williams D.A. Drug Metabolism. In: Lemke T.L., Williams D.A., Roche V.F., Zito S.W., editors. Foye’s Principles of Medicinal Chemistry. 7th ed. Wolters Kluwer and Lippincott Williams and Wilkins; London, UK: 2013. p. 135.
-
- Williams D.A., Roche V.E., Roche E.B. Central Analgesics, Opioid receptors. In: Lemke T.L., Williams D.A., Roche V.F., Zito S.W., editors. Foye’s Principles of Medicinal Chemistry. 7th ed. Wolters Kluwer and Lippincott Williams and Wilkins; London, UK: 2013. pp. 666–670.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

