A Human DPP4-Knockin Mouse's Susceptibility to Infection by Authentic and Pseudotyped MERS-CoV
- PMID: 30142928
- PMCID: PMC6164841
- DOI: 10.3390/v10090448
A Human DPP4-Knockin Mouse's Susceptibility to Infection by Authentic and Pseudotyped MERS-CoV
Erratum in
-
Correction: Fan et al. A Human DPP4-Knockin Mouse's Susceptibility to Infection by Authentic and Pseudotyped MERS-CoV. Viruses 2018, 10, 448.Viruses. 2025 Mar 3;17(3):363. doi: 10.3390/v17030363. Viruses. 2025. PMID: 40091792 Free PMC article.
Abstract
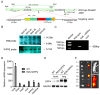
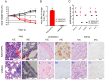
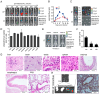
Infection by the Middle East respiratory syndrome coronavirus (MERS-CoV) causes respiratory illness and has a high mortality rate (~35%). The requirement for the virus to be manipulated in a biosafety level three (BSL-3) facility has impeded development of urgently-needed antiviral agents. Here, we established anovel mouse model by inserting human dipeptidyl peptidase 4 (hDPP4) into the Rosa26 locus using CRISPR/Cas9, resulting in global expression of the transgene in a genetically stable mouse line. The mice were highly susceptible to infection by MERS-CoV clinical strain hCoV-EMC, which induced severe diffuse pulmonary disease in the animals, and could also be infected by an optimized pseudotyped MERS-CoV. Administration of the neutralizing monoclonal antibodies, H111-1 and m336, as well as a fusion inhibitor peptide, HR2P-M2, protected mice from challenge with authentic and pseudotyped MERS-CoV. These results confirmed that the hDPP4-knockin mouse is a novel model for studies of MERS-CoV pathogenesis and anti-MERS-CoV antiviral agents in BSL-3 and BSL-2facilities, respectively.
Keywords: MERS-CoV; authentic virus; hDPP4; mouse model; pseudotyped virus.
Conflict of interest statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Therefore, they are no competing interests (financial or non-financial, professional, or personal) related to this work. Some mAbs were produced by Sino Biological Inc., which is an independent company with a long-term cooperative relationship with the National Institutes for Food and Drug Control. The company has been sufficiently informed of the work. They have no any financial or non-financial, professional, or personal competing interests, and they support the publication of this manuscript.
Figures



References
-
- Du L., Zhao G., Chan C.C., Sun S., Chen M., Liu Z., Guo H., He Y., Zhou Y., Zheng B.-J. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009;393:144–150. doi: 10.1016/j.virol.2009.07.018. - DOI - PMC - PubMed
-
- Zhao G., Du L., Ma C., Li Y., Li L., Poon V.K., Wang L., Yu F., Zheng B.-J., Jiang S. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol. J. 2013;10:266. doi: 10.1186/1743-422X-10-266. - DOI - PMC - PubMed
-
- De Wit E., Rasmussen A.L., Falzarano D., Bushmaker T., Feldmann F., Brining D.L., Fischer E.R., Martellaro C., Okumura A., Chang J., et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc. Natl. Acad. Sci. USA. 2013;110:16598–16603. doi: 10.1073/pnas.1310744110. - DOI - PMC - PubMed
-
- Raj V.S., Smits S.L., Provacia L.B., van den Brand J.M., Wiersma L., Ouwendijk W.J., Bestebroer T.M., Spronken M.I., van Amerongen G., Rottier P.J., et al. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J. Virol. 2014;88:1834–1838. doi: 10.1128/JVI.02935-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

