Targeting FcRn to Generate Antibody-Based Therapeutics
- PMID: 30143244
- PMCID: PMC6169532
- DOI: 10.1016/j.tips.2018.07.007
Targeting FcRn to Generate Antibody-Based Therapeutics
Abstract
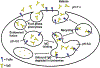
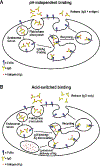
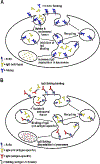
The MHC class I-related receptor FcRn serves multiple roles ranging from the regulation of levels of IgG isotype antibodies and albumin throughout the body to the delivery of antigen into antigen loading compartments in specialized antigen-presenting cells. In parallel with studies directed towards understanding FcRn at the molecular and cellular levels, there has been an enormous expansion in the development of engineering strategies involving FcRn to modulate the dynamic behavior of antibodies, antigens, and albumin. In this review article, we focus on a discussion of FcRn-targeted approaches that have resulted in the production of novel antibody-based platforms with considerable potential for use in the clinic.
Keywords: FcRn; FcRn-targeted therapeutics; antibody engineering; pharmacokinetics.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest disclosure
E.S.W. is a (co-)inventor on licensed patents related to half-life extension and Abdeg technology and has a financial interest in argenx. The licensed patents are owned by UT Southwestern Medical Center.
Figures

References
-
- Scott AM et al. (2012) Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287 - PubMed
-
- Goldstein R et al. (2013) Developments in single photon emission computed tomography and PET-based HER2 molecular imaging for breast cancer. Expert Rev. Anticancer Ther 13, 359373 - PubMed
-
- Wu AM (2009) Antibodies and antimatter: the resurgence of immuno-PET. J. Nucl. Med 50, 2–5 - PubMed
-
- Challa DK et al. (2014) FcRn: from molecular interactions to regulation of IgG pharmacokinetics and functions. Curr. Top. Microbiol. Immunol 382, 249–272 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

